UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2020
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-35776
ACASTI PHARMA INC.
(Exact name of registrant as specified in its charter)
| Québec, Canada | 98-1359336 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
545, Promenade du Centropolis, Suite 100, Laval, Québec H7T 0A3
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: 450-687-2262
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Name of each exchange on which registered | |
| Common Shares, no par value per share | NASDAQ Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common shares held by non-affiliates of the registrant, based on the closing sale price of the registrant’s common shares on the last business day of its most recently completed second fiscal quarter, as reported on the NASDAQ Stock Market, was approximately $161,005,499.55.
The number of outstanding common shares of the registrant, no par value per share, as of June 26, 2020 was 92,488,385.
ACASTI PHARMA INC.
FORM 10-K
For the Fiscal Year Ended March 31, 2020
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains information that may be forward-looking information within the meaning of Canadian securities laws and forward-looking statements within the meaning of U.S. federal securities laws, both of which we refer to in this annual report as forward-looking information. Forward-looking information can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking information in this annual report includes, among other things, information or statements about:
| · | our ability to conduct all required clinical and nonclinical trials for our drug candidate, CaPre, including the timing and results of those trials; | |
| · | the outcome of our ongoing dialogue with the U.S. Food and Drug Administration, or FDA, regarding the unusually large placebo effect observed in the triglyceride, or TG, topline results of our TRILOGY 1 Phase 3 clinical trial and the implications for our TRILOGY 2 Phase 3 clinical trial and its outcome; | |
| · | our ability to file a New Drug Application, or NDA, based on the results of our TRILOGY Phase 3 program; | |
| · | whether the FDA may require additional clinical development work or study to support an NDA filing for CaPre; |
| · | our strategy, future operations, prospects and the plans of our management; |
| · | the regulatory plan, timeline, costs and results of our clinical and nonclinical trials for CaPre; |
| · | the timing and outcome of our meetings and discussions with the FDA; |
| · | our planned regulatory filings for CaPre, and their timing; | |
| · | our expectation that our Bridging Study (as defined below) results will support our plan to get authorization from the FDA to use the 505(b)(2) pathway with new chemical entity, or NCE, status towards an NDA approval in the United States; |
| · | the potential benefits and risks of CaPre as compared to other products in the pharmaceutical, medical food, natural health and dietary supplement products markets; |
| · | our estimates of the size and growth rate of the potential market for CaPre, unmet medical needs in that market, the potential for future market expansion, the rate and degree of market acceptance of CaPre if it reaches commercialization, and our ability to serve that market; |
| · | our anticipated marketing advantages and product differentiation of CaPre and its potential to become a best-in-class omega-3, or OM3, compound for the treatment of severe hypertriglyceridemia, or sHTG; |
| · | the potential to expand CaPre’s indication for the treatment of high TGs (200-499 mg/dL), assuming at least one additional study; |
| · | the degree to which physicians would switch their patients to a product with CaPre’s target product profile based on the outcome of our TRILOGY Phase 3 trials; |
| · | our strategy and ability to develop, commercialize and distribute CaPre in the United States and elsewhere; |
| · | our ability to strengthen our patent portfolio and other means of protecting our intellectual property rights, including our ability to obtain additional patent protection for CaPre; |
| 1 |
| · | the availability and consistency of our raw materials, including raw krill oil, or RKO, from existing and future alternative suppliers; |
| · | our expectation that following expiration of our license agreement with Neptune Wellness Solutions Inc., or Neptune, we will not require any licenses from third parties to support the commercialization of CaPre; |
| · | our expectation to be able to rely on third parties to manufacture CaPre whose manufacturing processes and facilities are in compliance with current good manufacturing practices, or cGMP; |
| · | the potential for CaPre in other cardiometabolic medicine indications; |
| · | our intention and ability to build a U.S. commercial organization, and to successfully launch CaPre and compete in the U.S. market; |
| · | our intention and ability to complete development and/or distribution partnerships to support the commercialization of CaPre outside of the United States, and to pursue strategic opportunities to provide supplemental capital and market access; |
| · | the potential adverse effects that the recent COVID-19 pandemic may have on our business and operations; |
| · | our need for additional financing, and our estimates regarding our future financing and capital requirements; |
| · | our expectation regarding our financial performance, including our revenues, cost-of-goods, profitability, research and development, costs and expenses, gross margins, liquidity, capital resources, and capital expenditures; and |
| · | our projected capital requirements to fund our anticipated expenses, including our research and development, marketing and sales, general and administrative expenses, and capital equipment expenditures. |
Although the forward-looking information in this annual report is based upon what we believe are reasonable assumptions, you should not place undue reliance on that forward-looking information since actual results may vary materially from it. Important assumptions made by us when making forward-looking statements include, among other things, assumptions by us that:
| · | we are able to obtain the additional capital and financing we require when we need it; |
| · | the FDA will not require an additional study for us to file an NDA for CaPre, and that we successfully and timely complete all required clinical and nonclinical trials necessary for regulatory approval of CaPre; |
| · | the timeline and costs for our TRILOGY Phase 3 program are not materially underestimated or affected by the COVID-19 pandemic or other unforeseen circumstances; |
| · | CaPre is safe and effective; |
| · | we obtain and maintain regulatory approval for CaPre on a timely basis; |
| · | we are able to attract, hire and retain key management and skilled scientific and commercial personnel; | |
| · | third parties provide their services to us on a timely and effective basis; |
| · | we are able to maintain our required supply of raw materials at a reasonable price, including RKO; | |
| · | we are able to scale-up production of CaPre with third-party manufacturers to support commercial demand; |
| 2 |
| · | we are able to successfully build a commercial organization, launch CaPre in the United States, and compete in the U.S. market; |
| · | we are able to secure distribution arrangements for CaPre outside of the United States, if it reaches commercialization; |
| · | we are able to manage and fund our future growth effectively; |
| · | we are able to gain acceptance of CaPre in its targeted markets, and we are able to serve those markets; |
| · | our patent and trademark portfolio is sufficient and valid; |
| · | we are able to secure and defend our intellectual property rights, and to avoid infringing upon the intellectual property rights of third parties; |
| · | we are able to take advantage of new business opportunities in the pharmaceutical industry; | |
| · | we are able to execute on strategic partnerships according to our business plan; |
| · | we are able to continue as a going concern; |
| · | there is no significant increase in competition for CaPre from other companies in the pharmaceutical, medical food, dietary supplement and natural health product industries; |
| · | CaPre would be viewed favorably by payers at launch, and receive appropriate healthcare reimbursement; |
| · | market data and reports reviewed by us are accurate; |
| · | there are no material adverse changes in relevant laws or regulations; and |
| · | we face no product liability lawsuits or other proceedings or any such matters, if they arise, are satisfactorily resolved. |
In addition, the forward-looking information in this annual report is subject to a number of known and unknown risks, uncertainties and other factors, including those described in this annual report under the heading “Item 1A. Risk Factors”, many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking information, including, among others:
| · | risks related to timing and possible difficulties, delays or failures in our ongoing TRILOGY Phase 3 program for CaPre; |
| · | nonclinical and clinical trials may be more costly or take longer to complete than anticipated and may never be completed, or they may generate results that warrant future clinical trials, additional clinical development and/or delay commercialization of CaPre; | |
| · | our TRILOGY Phase 3 trials may not achieve all or any of its primary and secondary endpoints; | |
| · | assuming our TRILOGY 2 trial meets its primary endpoint, the results of pooling that data with our TRILOGY 1 trial results may not achieve statistical significance or, may not be supported by the FDA; | |
| · | based on the final TRILOGY 1 and TRILOGY 2 clinical trial data, the FDA may require that we conduct additional clinical work or studies to support an NDA for CaPre; |
| · | our anticipated studies and submissions to the FDA may not occur as currently anticipated, or at all; | |
| · | the FDA could reject our 505(b)(2) regulatory pathway and/or our NDA; |
| 3 |
| · | while the REDUCE-IT results (a cardiovascular outcome study conducted by Amarin Corporation plc, or Amarin, with their OM3 drug VASCEPA) were positive, on January 13, 2020, AstraZeneca plc announced that its cardiovascular Phase 3 STRENGTH trial for its OM3 drug EPANOVA had been discontinued due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidemia. The potential impacts of the discontinuance of the STRENGTH trial on our business and the OM3 drug market in general are not yet known; |
| · | if Amarin loses its appeal of the U.S. District Court for the District of Nevada’s March 30, 2020 decision invalidating its patent on the basis of obviousness, then additional generic versions of VASCEPA could potentially enter the market within the next year and this could result in downward pressure on pricing for CaPre; |
| · | we may encounter difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials or to market CaPre, or the FDA may refuse to approve CaPre or place restrictions on our ability to commercialize and promote CaPre; |
| · | the FDA may require, or for competitive reasons we may need to, conduct additional future clinical trials for CaPre, the occurrence and success of which cannot be assured; |
| · | CaPre may have unknown side effects, or may not prove to be as safe and effective or as potent as we currently believe; |
| · | CaPre could be subject to extensive post-market obligations and continued regulatory review, which may result in significant additional expense and affect sales, marketing and profitability; |
| · | we may fail to achieve our publicly announced milestones on time; |
| · | we may encounter difficulties in completing or funding additional development or commercialization of CaPre; | |
| · | third parties we are relying upon to conduct our TRILOGY Phase 3 program and support the data analysis and filing of an NDA for CaPre may not effectively fulfill their obligations to us, including complying with FDA requirements; |
| · | there may be difficulties, delays, or failures in obtaining health care reimbursements for CaPre; |
| · | recently enacted and future laws may increase the difficulty and cost for us to obtain marketing approval and commercialization of CaPre, and may affect the prices we can charge; |
| · | new laws, regulatory requirements, and the continuing efforts of governmental and third-party payors to contain or reduce the costs of healthcare through various means could adversely affect our business; |
| · | the market opportunity for, and demand and market acceptance of, CaPre may not be as strong as we anticipate; |
| · | third parties that we will rely upon to manufacture, supply and distribute CaPre may not effectively fulfill their obligations to us, including complying with FDA requirements; |
| · | there may not be an adequate supply of raw materials, including RKO, in sufficient quantities and quality to produce CaPre under cGMP standards and that meet our target specifications; |
| · | we may not be able to meet applicable regulatory standards for the manufacture of CaPre or scale-up our manufacturing successfully; |
| · | as a development stage company, we currently have limited sales, marketing and distribution personnel and resources; |
| 4 |
| · | our patent applications may not result in issued patents, our issued patents may be circumvented or challenged and ultimately struck down, and we may not be able to successfully protect our trade secrets or other confidential proprietary information; |
| · | we may not be able to build name recognition in our markets of interest if we do not protect our trademark for CaPre or any new trademark that is developed for CaPre; |
| · | we may face claims of infringement of third party intellectual property and other proprietary rights; |
| · | we may face product liability claims and product recalls; |
| · | we may face intense competition from other companies in the pharmaceutical, medical food and natural health product industries; |
| · | we have a history of negative operating cash flow, and may never become profitable or be able to sustain profitability; |
| · | we have significant additional future capital needs, and may not be able to raise additional financing required to fund further research and development, clinical studies, obtain regulatory approvals, build a commercial organization in the United States, and meet ongoing capital requirements to continue our current operations on commercially acceptable terms or at all; |
| · | we face additional costs related to the change in our status from a foreign private issuer to a U.S. domestic issuer; |
| · | we may not be able to successfully compete in the U.S. market with competitors who are larger and have more resources than we do; |
| · | we may acquire businesses or products or form strategic partnerships in the future that may not be successful; |
| · | we may be unable to secure development and/or distribution partnerships to support the development and commercialization of CaPre, provide development capital, or provide market access in any key market; |
| · | we rely on the retention of key management and skilled scientific, manufacturing, regulatory and commercial personnel; and |
| · | general changes in economic and capital market conditions could adversely affect us. |
All of the forward-looking information in this annual report is qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking information. Except as required by applicable law, we do not undertake to update or amend any forward-looking information, whether as a result of new information, future events or otherwise. All forward-looking information is made as of the date of this annual report.
We express all amounts in this annual report in U.S. dollars, except where otherwise indicated. References to “$” and “US$” are to U.S. dollars and references to “C$” or “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this annual report to “Acasti,” “the Company,” “we,” “us” and “our” refer to Acasti Pharma Inc. and its consolidated subsidiaries.
| 5 |
| Item 1. | Business |
Overview
We are a biopharmaceutical innovator focused on the research, development and commercialization of prescription drugs using OM3 fatty acids delivered both as free fatty acids and bound-to-phospholipid esters, derived from krill oil. OM3 fatty acids have extensive clinical evidence of safety and efficacy in lowering triglycerides, or TGs, in patients with hypertriglyceridemia, or HTG. Our lead product candidate is CaPre, an OM3 phospholipid therapeutic, which we are developing initially for the treatment of sHTG, a condition characterized by very high or severe levels of TGs in the bloodstream (≥ 500 mg/dL). In accordance with a study published in 2009 in the Archives of Internal Medicine by Ford et al., it is estimated that three to four million people in the United States have sHTG. In primary qualitative market research studies commissioned by Acasti in August 2016 and November 2017 by DP Analytics, a division of Destum Partners, and in April 2019 by a well-respected third party provider, key opinion leaders, or KOLs, high volume prescribers, or HVPs, and pharmacy benefit managers, or PBMs, who were interviewed indicated a significant unmet medical need exists for an effective, safe and well-absorbing OM3 therapeutic that can also demonstrate a positive impact on the major blood lipids associated with cardiovascular disease risk. We believe that CaPre may address this unmet medical need if our TRILOGY Phase 3 clinical program is successful in reproducing what we observed in our Phase 2 clinical data. See “—Our Clinical Data” and “—Our TRILOGY Phase 3 Program”.
We also believe the potential exists to expand CaPre’s initial indication to the roughly 44.4 million patients in the United States with elevated TGs in the mild to moderate range (e.g., blood levels between 200 - 499 mg/dL), although at least one additional clinical trial would likely be required to support FDA approval of a supplemental NDA to expand CaPre’s indication to this segment. Data from our Phase 2 studies indicated that CaPre may have a positive effect in diabetes and other inflammatory and cardiometabolic diseases; consequently, we may also seek to identify new potential indications for CaPre that may be appropriate for future studies and pipeline expansion. In addition, we may also seek to in-license other cardiometabolic or other primary care-focused drug candidates for drug development and commercialization.
In four clinical trials conducted to date, we saw the following consistent results with CaPre, and we are seeking to demonstrate similar safety and efficacy in our TRILOGY Phase 3 program:
| · | significant reduction of TGs and non-high density lipoprotein cholesterol (non-HDL-C) levels in the blood of patients with mild to sHTG; |
| · | no deleterious effect on low-density lipoprotein cholesterol (LDL-C), or “bad” cholesterol, with the potential to reduce LDL-C; |
| · | potential to increase high-density lipoprotein cholesterol (HDL-C), or “good” cholesterol; |
| · | potential to benefit diabetes patients by decreasing hemoglobin A1c (HbA1c), a marker of glucose control; |
| · | good bioavailability (absorption by the body), even under fasting conditions; |
| · | no significant food effect when taken with either low-fat or high-fat meals; and |
| · | an overall safety profile similar to that demonstrated by currently marketed OM3s. |
We believe that if we are able to reproduce these results in our TRILOGY Phase 3 program, that this could potentially set CaPre apart from current FDA-approved fish oil-derived OM3 treatment options, and it could give us a significant clinical and marketing advantage.
| 6 |
Recent Developments
As we have previously disclosed, we filed a Type C meeting request at the end of March 2020 with the FDA. We subsequently submitted our briefing package on April 29, 2020 to the FDA. The briefing package was intended to provide the FDA with a review of the relevant TRILOGY 1 clinical data and audit findings, with the objective of gaining alignment on the interpretation of the TRILOGY 1 results and implications for TRILOGY 2. We also sought the FDA’s input on our proposed revisions to the pre-specified TRILOGY 2 Statistical Analysis Plan, or SAP, and their input on a plan for pooling the data from TRILOGY 1 and TRILOGY 2 to support an NDA filing.
On June 19, 2020, we announced that the FDA had provided us with a written response to our meeting request and briefing package. The FDA confirmed that it will require pivotal efficacy analyses to be performed on the full Intent to Treat, or ITT, population as contemplated in the original SAP, and it supported the conduct of post-hoc analyses in TRILOGY 1 for exploratory purposes. Consistent with our prior disclosures and depending on the outcome of TRILOGY 2, an additional clinical study may still be needed prior to an NDA submission. We and our expert advisors are carefully considering the FDA’s comments on the TRILOGY 1 data and will conduct further post-hoc analysis based on the FDA’s feedback.
Based on the written feedback received from the FDA, we intend to now finalize the SAP for TRILOGY 2, which we plan to submit to the FDA by the end of July 2020. We continue to remain blinded to the TRILOGY 2 clinical data and we continue to expect to report topline data from TRILOGY 2 by the end of August 2020. The key secondary and exploratory endpoints from both TRILOGY 1 and TRILOGY 2 trials would still be expected as soon as possible after the unblinding of TRILOGY 2 results.
Additional details on our post-hoc data analyses of TRILOGY 1 results and clinical site and laboratory audit findings are summarized below, along with our planned next steps for unblinding TRILOGY 2 results. See “— TRILOGY 1 Findings based on Post-Hoc Analyses and Audits.”
On April 30, 2020, we announced that we had received notice of issuance of a composition of matter patent awarded by the Intellectual Property Office in Hong Kong. This new patent expands our intellectual property portfolio by granting claims for any composition containing eicosapentaenoic acid, or EPA, and docosahexaenoic acid, or DHA, where at least 50% of the composition consists of phospholipids.
About Hypertriglyceridemia (HTG)
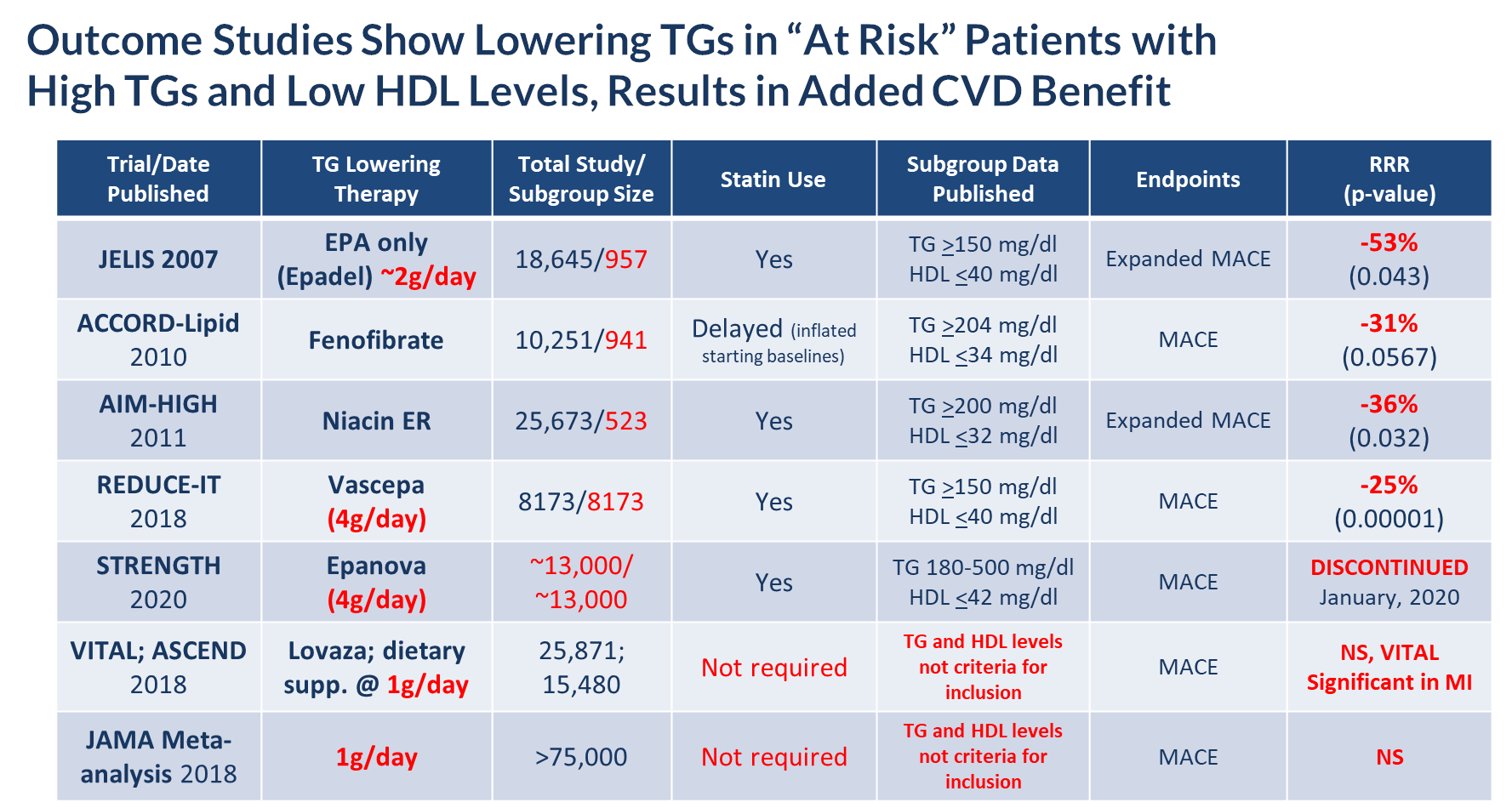
According to the American Heart Association Scientific Statement on Triglycerides and Cardiovascular Disease from 2011, TG levels provide important information as a marker associated with the risk for heart disease and stroke, especially when an individual also has low levels of HDL-C and elevated levels of LDL-C. HTG can be caused by both genetic and environmental factors, including obesity, sedentary lifestyle and high-fat diets. HTG is also associated with comorbid conditions such as chronic renal failure, pancreatitis, nephrotic syndrome, and diabetes. Multiple epidemiological, clinical, genetic studies suggest that patients with elevated TG levels (≥ 200 mg/dL) are at a greater risk of coronary artery disease, or CAD, and pancreatitis, a life-threatening condition, as compared to those with normal TG levels. The genes regulating TGs and LDL-C are equally strong predictors of CAD. Other studies suggest that lowering and managing TG levels may reduce these risks. In addition, the Japan EPA Lipid Intervention Study, or JELIS, demonstrated the long-term benefit of an OM3 EPA in preventing major coronary events in hypercholesterolemic patients receiving statin treatment. JELIS found a 19% relative risk reduction in major coronary events in patients with relatively normal TGs but a more pronounced 53% reduction in the subgroup of patients with TGs > 150mg/dL and HDL-C < 40mg/dL. Meta-analyses published by Alexander et al. (Mayo Clinic Proceedings, 2017) and Maki et al. (Journal of Clinical Lipidology, 2016) suggest that EPA and DHA may be associated with reducing coronary heart disease risk to a greater extent in populations with elevated TG levels, and that drugs lowering TG and TG-rich lipoproteins may reduce cardiovascular event risk in patients with elevated TG levels, particularly if associated with low HDL-C. In November 2018, Amarin published the results of its REDUCE-IT cardiovascular outcome trial, or CVOT, which showed that a therapeutic dose of VASCEPA at 4 grams per day, taken on top of a statin, reduced residual cardiovascular risk by 25%. Based on this data, in December 2019, the FDA granted Amarin an expanded label for VASCEPA that allows its use in patients with mild to moderate HTG (200 – 500mg/dL). The table below lists several CVOT studies done over approximately the last 13 years, and supports the hypothesis that the right dose of any drug (e.g. OM3, fibrate or niacin) that reduces TG levels in at risk patients (e.g. those with elevated TGs and low HDL-C), can significantly reduce their cardiovascular risk.
| 7 |

About CaPre
CaPre is a highly purified, proprietary krill oil-derived mixture containing polyunsaturated fatty acids, or PUFAs, primarily composed of OM3 fatty acids, principally EPA and DHA, present as a combination of phospholipid esters and free fatty acids. EPA and DHA are well known to be complementary and beneficial for human health, and according to numerous recent clinical studies, may promote healthy heart, brain and visual function (Kwantes and Grundmann, Journal of Dietary Supplements, 2014), and may also contribute to reducing inflammation and blood levels of TGs (Ulven and Holven, Vascular Health and Risk Management, 2015). Krill is a rich natural source of phospholipids and OM3 fatty acids. The EPA and DHA contained in CaPre are delivered as a combination of OM3s as free fatty acids and OM3s bound to phospholipid esters. Both forms allow these PUFAs to reach the small intestine where they undergo rapid absorption and transformation into complex fat molecules that are required for lipid transport into the bloodstream. We believe that EPA and DHA are more efficiently transported by phospholipids sourced from krill oil than the EPA and DHA contained in fish oil, which are transported either by TGs (as in dietary supplements) or as ethyl esters as in other prescription OM3 drugs (such as LOVAZA and VASCEPA). These OM3 ethyl ester prescription products must undergo additional digestion before they are ready for transport into the bloodstream. The digestion and absorption of OM3 ethyl ester drugs requires a particular enzymatic process that is highly dependent on the fat content of a meal – the higher the fat content, the better the OM3 ethyl ester absorption. High fat content meals are not recommended in patients with HTG. We believe that CaPre’s superior absorption profile could represent a significant clinical advantage, since taking it with a low-fat meal represents a healthier and more realistic regimen for patients with HTG who must follow a restricted low-fat diet. CaPre is intended to be used as a therapy combined with positive lifestyle changes, such as a healthy diet and exercise, and can be administered either alone or with other drug treatment regimens such as fibrates and/or statins (a class of drug used to reduce LDL-C). CaPre is intended to be taken orally once or twice per day in capsule form.
Potential Market for CaPre
We believe a significant opportunity exists for OM3 market expansion because, among other things:
| · | Cardiovascular diseases, or CVD, and stroke are the leading causes of morbidity and mortality in the United States. The burden of CVD and stroke in terms of life-years lost, diminished quality of life, and direct and indirect medical costs also remains enormous. According to the American Heart Association, in 2016, CVD cost the American healthcare system $555.0 billion. By 2035, this cost is estimated by the American Heart Association to increase to $1.1 trillion; |
| 8 |
| · | Evidence suggests potential for OM3s in other cardiometabolic indications, such as diabetes and high blood pressure; |
| · | Subgroup analyses from outcome studies conducted since 2007 such as JELIS, ACCORD-Lipid and AIM-HIGH, have all shown that patients who entered these studies with high TGs (above 150 mg/dL) and low HDL (below 40 mg/dL) and received a TG-lowering medication (either an OM3, fibrate or niacin) saw a relative cardiovascular risk reduction of 31 – 53% by the end of the study when compared to placebo or standard of care; and |
| · | In February 2019, following the release of Amarin’s REDUCE-IT results in September 2018, Cantor Fitzgerald projected that based on their market research survey with 100 physicians, prescriptions for OM3s were expected to grow significantly in 2019. Audited prescription data from Symphony Health Analytics showed that by August 2019, the U.S. market for OM3 therapeutics had reached an annualized run rate of more than $1.65 billion, up from $1.4 billion for the full year of 2018. |
According to the American Heart Association, the prevalence of HTG in the United States and globally correlates to the aging of the population and the increasing incidence of obesity and diabetes. Market participants, including the American Heart Association, have estimated that one-third of adults (approximately 70 million people) in the United States have elevated levels of TGs (TGs >150 mg/dL) (Ford, Archives of Internal Medicine, 2009; 169(6):572-578), including approximately 3 to 4 million people diagnosed with sHTG (Miller et al. Circulation, 2011 and Maki et al. J. Clan. Lipid, 2012). Moreover, according to Ford, Archives of Internal Medicine in a study conducted between 1999 and 2004, 18% of adults in the United States, corresponding to approximately 40 million people, had elevated TG levels equal to or greater than 200 mg/dL, of which only 3.6% were treated specifically with TG-lowering medication (Ford, Archives of Internal Medicine, 2009; 169(6):572-578; Kapoor and Miller, ACC, 2016, Christian et al. Am. J. Cardiology, 2011). We believe this data indicates there is a large underserved market opportunity for CaPre.
CaPre’s target market in the United States for treatment of HTG was estimated by Symphony Health Analytics Audit data to be approximately $1.4 billion in 2018, with approximately 4.5 million prescriptions written annually. The total global market for treatment of HTG was estimated by GOED Proprietary Research in 2015 to be approximately $2.3 billion annually. Until late 2019, all marketed OM3 products had been approved by the FDA only for patients with sHTG. On December 13, 2019, the FDA granted Amarin an expanded label for patients with TG levels above 150mg/dL, who also have established CVD or diabetes, and two or more additional risk factors for CVD, based upon the results of their REDUCE-IT outcome study. Given this expanded labeling for VASCEPA, we believe there is the potential to greatly expand the treatable market for OM3s in the United States to the approximately 70 million people with TGs above 150 mg/dL. It is not yet known whether the discontinuance by AstraZeneca of its Phase 3 STRENGTH CVOT for its OM3 drug EPANOVA (announced on January 13, 2020) will have an adverse effect on the size or growth rate of this potential treatable market. The REDUCE-IT and STRENGTH CVOT studies were designed to evaluate the long-term benefit of lowering TGs on CVD risk with prescription drugs containing OM3 fatty acids in patients with mild to moderately elevated TGs, low HDL-C, and concurrently taking a statin. Additional clinical trials would likely be required for CaPre to also expand its label claims to this segment.
CaPre currently has two FDA-approved and marketed branded competitors, LOVAZA and VASCEPA. Generic LOVAZA became available on the U.S. market in 2013. In spite of generic LOVAZA options, 2017 audited prescription data from IMS NSP indicates that approximately 70% of OM3 prescriptions were written for branded products (predominantly VASCEPA). According to Symphony Health Analytics Audit data from August 2019, the U.S. OM3 market for HTG is valued at more than $1.65 billion. However, the number of prescriptions written for branded OM3s is now increasing significantly since Amarin announced its REDUCE-IT results in late 2018 and has recently received expanded label claims. Normalized prescription growth for Amarin’s VASCEPA grew by 78% in 2019 compared to 2018. According to Amarin, they have forecasted net revenue in 2020 of $650 million to $700 million, mostly from sales of VASCEPA in the United States. However, if Amarin loses its appeal of the U.S. District Court for the District of Nevada’s March 30, 2020 decision invalidating its patent on the basis of obviousness, then additional generic versions of VASCEPA could enter the market in the next few years.
We conduct market research at least annually with physicians and payers to monitor market developments, reimbursement and clinical practice. Except as otherwise indicated, all of the information that follows under this section has been derived from secondary sources, including audited U.S. prescribing data, and from qualitative U.S. primary market research with physicians and payers conducted for us by Destum, and more recently by a well-respected third party survey provider.
| 9 |
Destum utilized secondary market data and reports to develop market projections for us, and they also conducted primary qualitative market research with physicians and third-party payers, such as pharmacy benefit managers, or PBMs. One-on-one in-depth phone interviews conducted in November 2017 lasting on average 30-60 minutes were conducted with 22 physicians and 5 PBMs. Key insights and data were collected by Destum on current clinical practice for treating patients with HTG, and physician and payer perceptions of the current unmet medical and key economic needs in this space. All interviews were conducted by the same individual at Destum to ensure consistency in the collection of key information. Destum utilized OM3 prescription data from 2009 to 2017 to estimate the size of CaPre’s potential market. Based on discussions with the PBMs, Destum also assumed CaPre would be viewed favorably by payers at launch (e.g., Tier 2 or 3, depending on payer plan, which is comparable to LOVAZA and VASCEPA) provided CaPre is similarly priced. Upon completing the screening questionnaire and being approved for inclusion in Destum’s study, key opinion leaders, or KOLs, and high volume prescribers, or HVPs, were provided with a study questionnaire and were asked to comment on a target profile for a potential new OM3 “Product X” delivering a “trifecta” of cardio-metabolic benefits similar to the potential efficacy and safety benefits demonstrated by CaPre in our two Phase 1 pharmacokinetic studies and two Phase 2 clinical trials, which we refer to as the “Target Product Profile.” Respondents were told that the unidentified product was being prepared for a Phase 3 program designed to confirm with statistical significance the product’s safety and efficacy in patients with sHTG. The Target Product Profile was used by Destum strictly for market research analysis purposes and should not be construed as an indication of future performance of CaPre and should not be read as an expectation or guarantee of future performance or results of CaPre, and will not necessarily be an accurate indication of whether or not such results will be achieved by CaPre in our TRILOGY Phase 3 program.
In the market research conducted for us, KOLs and HVPs interviewed by Destum were asked to assess the level of unmet medical need associated with treating patients with sHTG based on currently available treatment options. 91% of physicians interviewed by Destum in 2016 indicated that they believe that the current unmet medical need for treating HTG was moderate to high. That number increased to 100% in the subsequent December 2017 research. The reasons identified by these physicians for their dissatisfaction with the currently available OM3s included insufficient lowering of TGs (a complaint principally related to VASCEPA), negative LDL-C effects (a complaint principally related to LOVAZA), the “food effect” or reduced absorption of both LOVAZA and VASCEPA when taken with a low-fat meal (or the corollary to this concern, which is that their patients had to take either drug with a fatty meal to get full efficacy benefit), gastrointestinal side effects, and the fishy taste from these fish oil-derived OM3s. Physicians reported that their patients have difficulty swallowing the large 1 gram softgel capsules of VASCEPA and LOVAZA, and they worried about these issues contributing to patient non-compliance. Despite the availability of other drug classes to treat sHTG, interviewed physicians indicated that they would welcome the introduction of new and improved OM3 products, particularly if they can address these perceived deficiencies.
Interviewed physicians responded favorably to the blinded Target Product Profile of CaPre in the Destum Market Research studies. In the study conducted in December 2017, they indicated that they would prescribe a new OM3 drug with the Target Product Profile to approximately 82% of their patients in the sHTG patient population and 68% of their patients in the high HTG segment within two years of the new OM3 drug’s approval. Approximately 60% of the interviewed physicians indicated that they would switch to a drug with the Target Product Profile primarily due to the “trifecta effect” of reducing TGs and LDL-C while elevating HDL-C, and the remaining 40% indicated they would switch primarily due to a drug with the Target Product Profile due to the effective reduction of TGs alone. In connection with their responses, the interviewed physicians were instructed to assume the drug with the Target Product Profile and all currently available OM3 products were priced similarly and not subject to any reimbursement or coverage hurdles (e.g., all products were on an equal health care coverage playing field). This assumption was subsequently supported by our interviews with leading PBMs in the United States.
This market research was updated again in March 2019 to reflect the more current views of physicians and third party payers following the publication of the REDUCE-IT study results. This updated primary qualitative market research project was conducted by a well-respected third party survey provider, and the design of the study was similar to the Destum project, with one-on-one interviews lasting approximately 60 minutes in duration. These interviews were conducted with 10 physicians and 20 pharmacy directors, covering 179,913,005 commercial lives across the United States, consistent with the current payer mix for the OM3 market. CaPre was evaluated positively by physicians with particular value placed on its potential to lower TGs, LDL-C and HbA1c (this was seen as unique, and especially valued), and to increase HDL-C, as well as its potentially superior tolerability features (e.g., easier to swallow when compared to the ethyl ester fish oils, and no fishy taste or “burpiness”). On average, physicians indicated that they would begin prescribing CaPre 3 months after launch, and would evaluate its performance in their initial patients after 3 to 6 months of use. Depending on favorable experience in initial use, some physicians indicated peak use could begin as quickly as 12 to 18 months after launch. Physicians expect CaPre to be priced similar to VASCEPA, and to have an out-of-pocket cost after insurance reimbursement of approximately $10-$50. Payers also viewed CaPre favorably, and did not anticipate any major reimbursement restrictions, with likely coverage at Tier 2 or 3 depending on the payer plan.
| 10 |
The Redbook published by Thomson Reuters is widely used by healthcare professionals to assess the latest drug product pricing and packaging information on prescription and over-the-counter drug products. Based on recent Redbook pricing data from May 5, 2020, the average wholesale pricing for branded VASCEPA is currently approximately US$397 per month. Amarin has raised prices for VASCEPA annually since its launch in late 2013. PBMs typically offer “Preferred Brand” status (Tier 2 or Tier 3) for VASCEPA. By the end of 2018, VASCEPA had reached about 45% market share in the United States, in spite of generic competition from LOVAZA. Amarin continues to gain market share in the United States and, as of August 2019 based on Symphony Health Analytics prescription audit date, Amarin had reached about 64% of market share based on dollars, and had about 53% of market share based on units. This growth is principally coming from market expansion along with some erosion of generic sales.
We plan to continue to regularly conduct additional market research with KOLs, HVPs, primary care physicians and payers to further develop and refine our understanding of the potential market for CaPre ahead of potential commercial launch in the United States.
Our Nonclinical Research
In addition to our Phase 2 and 3 clinical trials, we carried out an extensive nonclinical program to demonstrate the safety of CaPre in a defined set of studies required by the FDA. These studies were carried out by contract research organizations in compliance with the FDA’s Good Laboratory Practices, or GLP, and conducted on various species of animals recommended by the FDA to investigate the long-term effects of CaPre at doses of up to 65 grams of human equivalent dose over 39 weeks. In these studies, hematological, biochemical, coagulation and overall health parameters of CaPre were evaluated and no toxic effects were observed in any of the segments of the studies. Other studies focused on the potential toxic effects of CaPre on vital systems, such as the cardiovascular, respiratory and central nervous system, as evaluated by behavioral studies of the various species. These studies showed that CaPre did not have any adverse or toxic effects on any of the vital systems investigated, again up to doses well above the equivalent recommended clinical dose of CaPre. To rule out short term toxic effects of CaPre on genes, genomic toxicity studies were undertaken on accepted cellular and animal models. These studies showed no toxic effects of CaPre on any of the genetic markers indicative of potential gene altering toxic effects.
We believe the studies conducted to date indicate that CaPre is well-tolerated and shows no toxic effects on any of the physiological and vital systems of the tested animals or their genes at doses well above CaPre’s anticipated clinical therapeutic dose of 4 grams daily.
In parallel to our TRILOGY Phase 3 program, we also conducted additional nonclinical studies, including a pre- and postnatal development study in rodents and a 26-week oral carcinogenicity study in transgenic homozygous rasH2 mice. Both study protocols were designed to support an NDA filing for CaPre and were pre-approved by the FDA by means of a special protocol assessment through the FDA’s Executive Carcinogenicity Assessment Committee. Both studies have now been completed and there was no evidence of a carcinogenic potential of NKPL66, which is CaPre’s active pharmaceutical ingredient, or API, in the transgenic Hemizygous rasH2 mice following daily oral gavage at doses up to 2000 mg/kg/day. In addition, administration of NKPL66, once daily oral gavage, was well tolerated in F0 female rats with no evidence of maternal toxicity and no effects on maternal performance. In addition, there were no effects on the development of F1 generation.
In addition to the non-clinical studies described above, which are required to support NDA filing, we also conducted an additional non-clinical study in mice to gain additional insights into CaPre’s potentially unique mechanism of action in diabetes. In our Phase 2 studies in humans, a statistically significant reduction of hemoglobin A1c (HbA1c) was seen in the 4 gram treatment arm of our COLT Phase 2 clinical trial. This is the same dose that is currently being tested in our TRILOGY Phase 3 program in humans. This positive HbA1c result in our COLT phase 2 clinical trial was unexpected at the time, and potentially unique to CaPre, as other therapeutic OM3s had previously shown a range of outcomes, from no effect to a potentially deleterious effect, on glucose metabolism in diabetic or pre-diabetic patients. The main objective for this mechanistic diabetes mouse study was to assess if CaPre acts on glucose and/or insulin in some unique manner, and to compare results head-to-head with icosapent ethyl (VASCEPA) and metformin, a widely-prescribed diabetic medication. We collaborated with Dr. André Marette, who is the Director of the Pfizer Chair to study the pathogenesis of insulin resistance and cardiometabolic diseases at the University Laval, Quebec, and conducted the study in widely used and well accepted animal models in diet-induced obese C57BL6 and dyslipidemic LDLrKO mice to compare the mechanisms of action of CaPre versus icosapent ethyl and metformin on insulin resistance and type 2 diabetes. Dr. Marette is a widely-published researcher of cardiometabolic disease.
| 11 |
The preliminary findings obtained for the diabetes mouse study showed that CaPre may promote insulin secretion as seen by statistically significant results produced in a standard glucose challenge test, thus suggesting a mechanism of action different and unique when compared to metformin, which does not promote insulin secretion. Furthermore, icosapent ethyl showed no effect on insulin or any improvement in glucose metabolism or management. Key additional findings from this diabetic mouse study are:
| · | CaPre increased insulin production in association with increased c-peptide levels, suggesting that this effect is linked to greater insulin secretion by ß cells. This effect was also associated with a tendency for lower glucose responses during a glucose challenge test. CaPre exhibited a dose response, where the higher the dose the more insulin was secreted. |
| · | Both CaPre and icosapent ethyl significantly increased plasma 18RS-HEPE, (a metabolite of EPA and a precursor of Resolvin E1) as compared to the untreated control and metformin groups. Despite the lower levels of EPA in CaPre’s composition, the actual levels of 18RS-HEPE reached in the blood were higher for CaPre than levels produced by icosapent ethyl. Again, a dose response effect was seen with CaPre. 18RS-HEPE and Resolvin E1 are both resolving mediators of OM3s, and particularly EPA, and they are involved in the resolution of inflammation that is triggered in many chronic diseases, including obesity and diabetes. |
| · | Both high-dose (human equivalent dose of 4 grams/day), and low-dose (human equivalent dose of 2 grams/day) of CaPre significantly increased plasma levels of 17S-HDHA and PDX (two metabolites of DHA) as compared to the untreated control group. The effects of high-dose CaPre on PDX was very robust and significant, and much greater than those of icosapent ethyl, which showed virtually no response. Research has shown that increased levels of PDX improves insulin sensitivity in various models of insulin resistance and diabetes by several mechanisms, including by limiting inflammation in metabolic tissues, as well as by enhancing skeletal muscle IL-6 secretion, AMP activated protein kinase activation and glucose uptake, and by enhancing insulin's ability to suppress hepatic glucose production, which is also elevated in diabetic patients. |
Data from the diabetic mouse study are still being compiled and finalized. A second study is underway in a fatty liver/NASH disease model to further confirm the findings of the diabetes study, and may potentially provide more insight into the mechanism of action of CaPre on the plasma lipid profile, and in fatty liver disease by further comparing the impact of CaPre on plasma TGs, LDL-C and HDL-C, as well as on hepatic lipid accumulation versus that of icosapent ethyl and metformin. We have also filed additional patents covering unique aspects and new potential therapeutic applications of this expanded understanding of CaPre’s mechanism of action.
Our Clinical Data
CaPre is being developed for the treatment of patients with sHTG. In two Phase 2 clinical trials conducted by us in Canada (our COLT and TRIFECTA trials), CaPre was well-tolerated at all doses tested, with no serious adverse events that were considered treatment-related. Among the reported adverse events with an occurrence of greater than 2% of subjects and greater than placebo, only diarrhea had an incidence of 2.2%.
In both Phase 2 clinical trials, CaPre significantly lowered TGs in patients with mild to sHTG. Importantly, in these studies, CaPre also demonstrated no deleterious effect on LDL-C (unlike LOVAZA and EPANOVA, which had been shown to significantly increase LDL-C in patients with sHTG). Further, our Phase 2 data indicated that unlike LOVAZA, CaPre may actually reduce LDL-C with a 4 gram per day dose (a dose equivalent to VASCEPA and LOVAZA). LDL-C is undesirable because it accumulates in the walls of blood vessels, where it can cause blockages (atherosclerosis). Clinically, the phospholipids may potentially not only improve the absorption, distribution, and metabolism of OM3s, but they may also decrease the synthesis of LDL cholesterol in the liver, impede or block cholesterol absorption, and stimulate lipid secretion from bile. In the Phase 2 trials, CaPre also significantly reduced non-HDL-C (all cholesterol contained in the bloodstream except HDL-C), which is also considered to be a marker of cardiovascular disease. The COLT trial data showed a mean increase of 7.7% in HDL-C with CaPre at 4 grams per day (p=0.07). Further analysis of the data from our TRILOGY Phase 3 program will be required to demonstrate CaPre’s statistical significance with respect to lowering LDL-C and increasing HDL-C. Finally, we saw a statistically significant reduction of HbA1c in the CaPre 4g treatment group in the COLT study after only 8 weeks on drug in a diabetic population of patients with HbA1c levels at or below 7.0% at baseline. This interesting and potentially differentiating effect is being investigated more thoroughly in our TRILOGY Phase 3 program, where a larger proportion of the patients are diabetic, with HbA1c levels up to 9.5%, and they will be followed for 6 months.
| 12 |
We believe that these multiple potential cardiometabolic benefits, if confirmed in our TRILOGY Phase 3 program, could be significant differentiators for CaPre in the marketplace, as no currently approved OM3 drug has shown an ability to positively modulate all four of these important blood lipids (TGs, non-HDL-C, LDL-C and HDL-C) in the treatment of patients with dyslipidemia. We also believe that if supported by additional clinical trials, CaPre has the potential to become the best-in-class OM3 compound for the treatment of mild to moderate HTG.
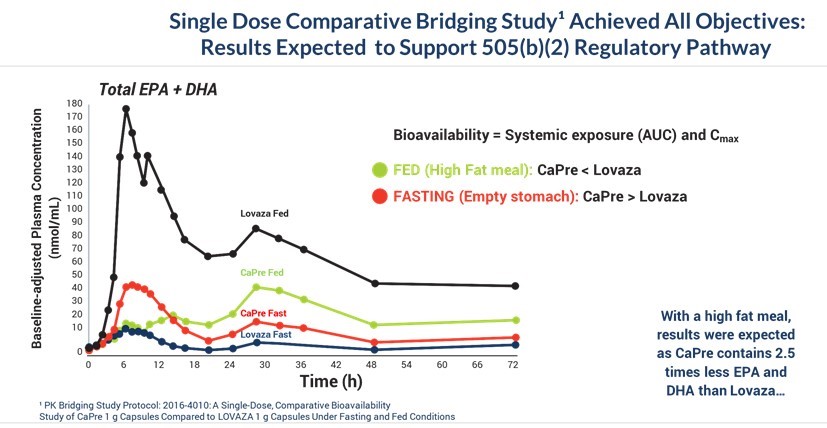
On September 14, 2016, we announced positive data from our completed comparative bioavailability study, or the “Bridging Study”. The Bridging Study was an open-label, randomized, four-way, cross-over, bioavailability study comparing CaPre, given as a single dose of 4 grams in fasting and fed (high-fat) states, as compared to the FDA-approved HTG drug LOVAZA (OM3-acid ethyl esters) in 56 healthy volunteers. The protocol was reviewed and approved by the FDA. The primary objective of the Bridging Study was to compare the bioavailability of CaPre to LOVAZA, each administered as a single 4-gram dose with a high-fat meal, which is the condition under which administration of OM3 drugs will yield the highest levels of EPA and DHA in the blood, and therefore has the highest potential for toxicity. For us to rely on the long-term safety data of LOVAZA to support a 505(b)(2) NDA for CaPre, our results had to show that the blood levels of EPA and DHA resulting from a single 4-gram dose of CaPre, are not significantly higher than those from a single 4-gram dose of LOVAZA under fed (high-fat meal) conditions. The Bridging Study met all of its objectives and demonstrated that the levels of EPA and DHA following administration of CaPre did not exceed corresponding levels following administration of LOVAZA in subjects who were fed a high-fat meal. We expect that these results will support a claim by us that CaPre and LOVAZA have a comparable safety profile. Also, among subjects in a fasting state, CaPre demonstrated better bioavailability than LOVAZA, as measured by significantly higher blood levels of EPA and DHA. Since most HTG patients must follow a restricted low-fat diet, we believe that CaPre’s strong bioavailability profile could provide a more effective clinical solution for these patients.
We summarized and submitted data from our Bridging Study to the FDA for review and discussed it with the FDA at an End of Phase 2 meeting during the first quarter of 2017. We also presented our Bridging Study data at the National Lipid Association Conference in May 2017, and this data was subsequently published in the peer-reviewed Journal of Clinical Therapeutics in 2019. The graph below illustrates that the Bridging Study achieved all of its objectives:
| 13 |
Absorption of EPA and DHA as ethyl ester formulations in the currently available prescription OM3 drugs derived from fish oil (such as LOVAZA and VASCEPA) requires the breakdown of the ethyl esters by pancreatic enzymes (lipases) to be released into the blood. These particular enzymes are produced in response to the consumption of high-fat content meals, leading to optimal absorption of DHA and/or EPA. As a result, these OM3 ethyl ester formulations have demonstrated lower absorption and bioavailability when taken with a low-fat meal or on an empty stomach. As shown in our CAP13-101 study described further below, absorption of CaPre, which is formulated as a combination of OM3 phospholipids and free fatty acids, is not meaningfully affected by the fat content of a meal consumed prior to drug administration. Since a low-fat diet is typically a critical component for treatment of patients with sHTG, we believe that being able to effectively combine CaPre with a low-fat diet could give CaPre a significant clinical and marketing advantage over the ethyl ester-based OM3s, such as LOVAZA and VASCEPA, that must be taken with a high-fat meal to achieve optimal absorption.
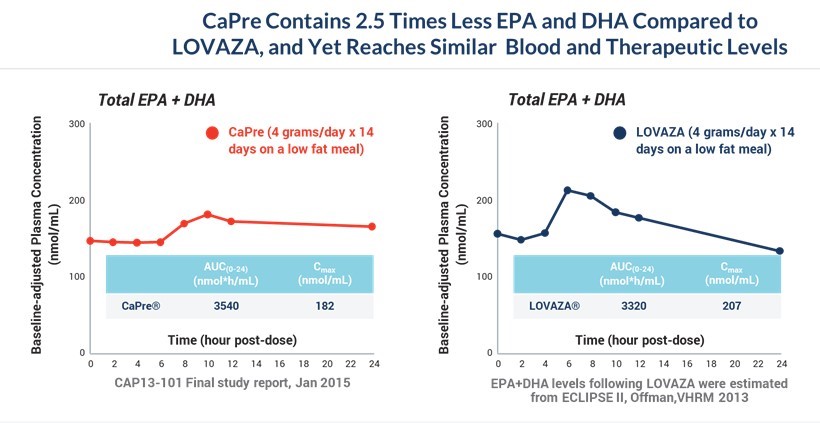
Our CAP13-101 study was an open-label, randomized, multiple-dose, single-center, parallel-design study in healthy volunteers. 42 subjects were enrolled into 3 groups of 14 subjects who took 1 gram, 2 grams or 4 grams of CaPre, administered once a day, 30 minutes after breakfast. The objectives of the study were to determine the pharmacokinetic, or PK, profile and safety on Day 1 following a single oral dose and Day 14 following multiple oral doses of CaPre in individuals pursuing a low-fat diet (therapeutic lifestyle change diet). The effect of a high-fat meal on the bioavailability of CaPre was also evaluated at Day 15. Blood samples were collected for assessment of EPA and DHA total lipids in plasma to derive the PK parameters.
The PK profile of CaPre following multiple 4-gram doses obtained in the CAP13-101 study at Day 14 was compared to the results obtained in a similar PK study (Offman 2013 - ECLIPSE 2) where LOVAZA was also administered at 4 grams a day for 14 days with a low-fat diet. Although CaPre contains approximately 2.5 times less EPA and DHA compared to LOVAZA (approximately 310 mg/1g capsule for CaPre versus 770 mg/1g capsule for LOVAZA), when administered with a low-fat meal, CaPre plasma levels of EPA and DHA are very similar to those of LOVAZA. This is indicated by the area under the plasma drug concentration against time curve, or AUC, and the maximal plasma drug concentration. This study gives us confidence in the dosing and design of our TRILOGY Phase 3 program, as we believe blood levels of EPA and DHA should translate into efficacy of TG reduction. Our CAP 13-101 study gives us confidence that 4 grams/day of CaPre could be as effective in lowering TGs as LOVAZA. We anticipate that our TRILOGY Phase 3 clinical program will confirm if this hypothesis is correct.
As illustrated by the two graphs below, CaPre reached similar blood and therapeutic levels to LOVAZA after 14 daily doses of CaPre at 4 grams/day, despite CaPre containing 2.5 times less EPA and DHA compared to LOVAZA:
| 14 |
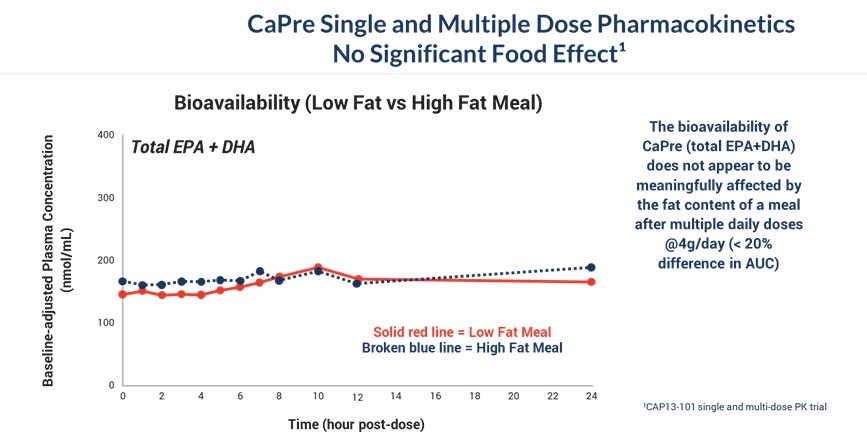
The graph below illustrates that the bioavailability of CaPre (total EPA+DHA levels in the blood) does not appear to be meaningfully affected by the fat content of a meal after multiple daily doses of CaPre at 4 grams/day (< 20% difference in AUC). We believe that CaPre’s strong bioavailability could represent a significant clinical advantage since taking it with a low-fat meal represents a more realistic and attractive regimen for patients with HTG who must follow a restricted low-fat diet.
Our CAP13-101 Study for CaPre Pharmacokinetics Shows No Significant Food Effect
The graph below presents a summary of the effects of CaPre on patient lipid profiles as obtained in our completed TRIFECTA and COLT Phase 2 clinical trials. 90% of the patients in these clinical trials had mild to moderate HTG (levels between 200 - 499 mg/dL) and only 10% of patients had sHTG (levels between 500 and 877 mg/dL), which was the maximum level of TGs permitted by Health Canada’s study protocol. Only 30% of the participating patients were taking statins, which we believe is important because statins appear to enhance the TG-lowering effect of OM3s. In contrast, in our competitors’ summary data that follows, 100% of the patients in those studies with mild to moderate HTG were taking statins with their OM3s.
The summary data from our COLT and TRIFECTA clinical trials shows that CaPre significantly reduces TGs, but unlike some other prescription EPA/DHA-based OM3s, it has no deleterious effect on LDL-C and may potentially increase HDL-C (p=0.07), which we refer to as the “trifecta effect”. Also, a dose response was seen for all of the major lipid markers; the greater the dose of CaPre, the greater the beneficial effect of CaPre.
Our Phase 2 Study Results Show CaPre Dose Response and Potential for “Trifecta” Lipid Effect
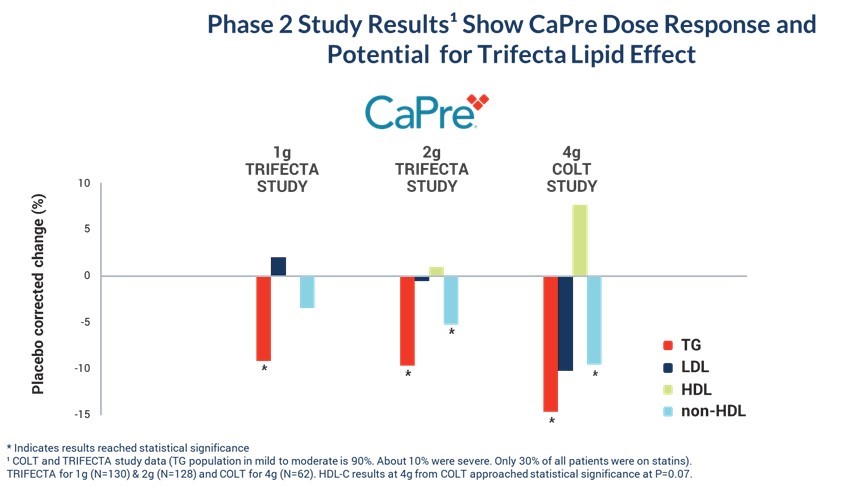
* Indicates results reached statistical significance
TRIFECTA for 1g (N=130) & 2g (N=128) and COLT for 4g (N=62). HDL-C results at 4g from COLT approached statistical significance at P=0.07.
| 15 |
We conducted a subgroup analysis including only patients with sHTG, consisting of approximately 10% of the patients from our TRIFECTA study, to compare the effects of CaPre versus other OM3 drugs in the initial target population of patients with sHTG. Despite being given at a lower dose (only 1 gram and 2 grams), CaPre’s results compared very well with data from independent studies for the other prescription OM3 drugs that are FDA-approved for the treatment of sHTG at higher doses of 2 grams and 4 grams. While the results of this subgroup analysis were not statistically significant for CaPre (potentially due to the small sample size), numerically, the results compared well with the other OM3 drugs, even though CaPre was given at a much lower dose. The results for LDL-C, HDL-C and non-HDL-C levels in the subgroup shown in the table below are based on descriptive statistics only and are solely directional, meaning that no statistical testing was conducted, and so no “p” values were generated. Note also that VASCEPA’s TG-lowering results from Amarin’s MARINE study were inflated due to a significant placebo effect that increased TGs in the placebo group as compared to baseline levels. This resulted in VASCEPA’s placebo-corrected TG reduction being overstated by about 10%.
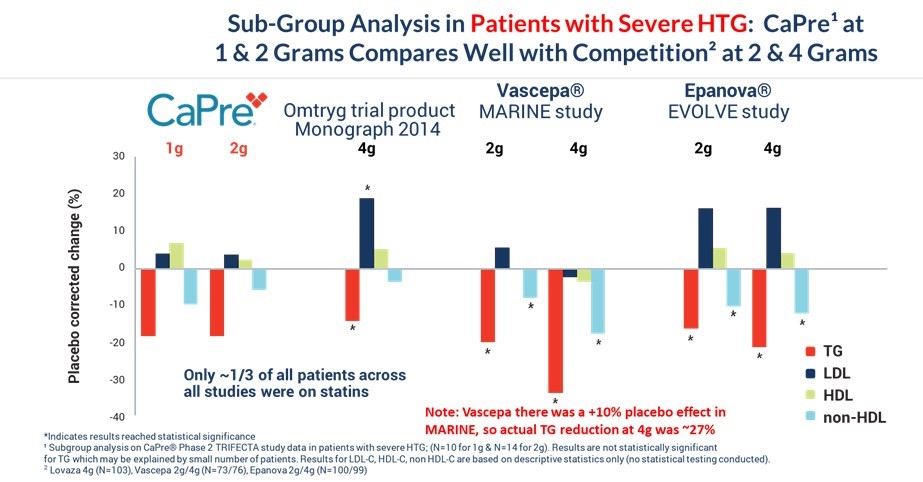
Since statins appear to enhance the TG-lowering property of OM3 drugs, we conducted a subgroup analysis that only included patients who were taking a statin at baseline in the COLT and TRIFECTA studies (approximately 30% of the population of both trials, combined). The graph below compares the TG-lowering effects of CaPre to other OM3s, all on a background of a statin drug, and shows that CaPre’s TG-lowering effects compare well with other FDA-approved OM3 drugs. We believe it is noteworthy that only 39 patients on 2 grams of CaPre in our TRIFECTA study (out of a total of 128) and only 22 patients on 4 grams of CaPre in our COLT study (out of 62) were taking statins.
The CaPre 2-gram bar graph in the table below shows the results from patients in our TRIFECTA trial who were taking statins. A statistically significant reduction in TGs (-25.7% placebo-corrected) was seen in that statin subgroup. The CaPre 4-gram bar graph in the table below shows patient results only from our COLT trial (as there was no 4-gram component for our TRIFECTA trial). None of the results were statistically significant at 4 grams of CaPre, potentially due to the small number of patients (22) in the statin subgroup.
As seen in the larger full study analyses in the tables above, CaPre does not show any deleterious effect on LDL, and shows the potential to decrease LDL and increase HDL (p=0.07). These observations will need to be confirmed in our TRILOGY Phase 3 program.
| 16 |
VASCEPA’s TG-lowering results from Amarin’s ANCHOR study were also inflated due to the use of mineral oil in their placebo group, which resulted in an increase of TG over baseline. This resulted in VASCEPA’s placebo-corrected TG reduction being overstated by about 6% in this study.
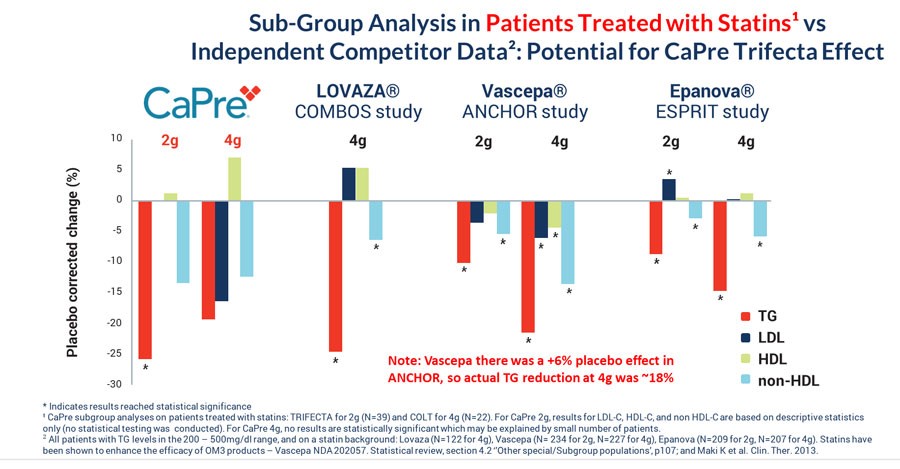
In summary, in addition to effectively reducing TG levels in patients with mild to sHTG, clinical data collected by us to date indicates that CaPre may also have:
| · | beneficial clinical effects on other blood lipids, such as HDL-C (good cholesterol) and non-HDL-C; |
| · | no deleterious effect on, and may potentially reduce, LDL-C (bad cholesterol) levels; |
| · | potential to benefit diabetes patients by reducing HbA1c, an important marker of diabetes; and |
| · | absorption capability that, unlike VASCEPA and LOVAZA, is not meaningfully affected by the fat content of a meal consumed prior to drug administration, providing patients with the reassurance that following their physician-recommended low-fat diet will still result in high absorption. |
We believe that these features could set CaPre apart from currently available FDA-approved OM3 treatment options in the marketplace and could give us a significant clinical and marketing advantage.
| 17 |
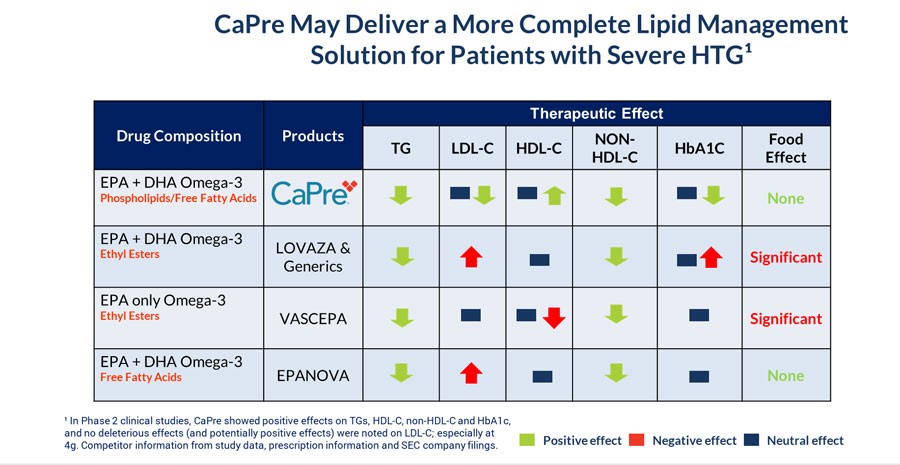
CaPre’s potential clinical benefits as compared to currently available FDA-approved OM3 treatment options are summarized in the table below and indicate that CaPre may deliver a more complete lipid management solution for patients with sHTG:
Our TRILOGY Phase 3 Program
In March 2017, we announced our plans to proceed with our TRILOGY Phase 3 program following our End-of-Phase 2 meeting with the FDA in February 2017. Based on the guidance we received from the FDA, we implemented two pivotal, randomized, placebo-controlled, double-blinded Phase 3 studies to evaluate the safety and efficacy of CaPre in patients with sHTG. These 26-week studies are evaluating CaPre’s ability to lower TGs from baseline in approximately 500 patients (approximately 250 per study) randomized to either 4 grams daily or placebo. The FDA’s feedback supported our plan to conduct two studies in parallel, potentially reducing the cost and shortening the time to an NDA submission. These studies were conducted in approximately 125 sites across North America.
The primary endpoint of these studies is to determine the efficacy of CaPre at 4 grams/day compared to placebo in lowering TGs after 12 weeks in sHTG patients, and to confirm safety and persistence of TG-lowering effect by following these patients for the full 26 weeks. The study was designed to provide at least 90% statistical power to detect a difference of at least a 20% decrease from baseline in TGs between CaPre and placebo. In addition, the TRILOGY Phase 3 studies included numerous secondary and exploratory endpoints, which are designed to assess the effect of CaPre on the broader lipid profile and certain metabolic, inflammatory and CVD risk markers.
In November 2017, we announced that Dariush Mozaffarian, M.D., Dr.P.H., agreed to serve as the principal investigator of our TRILOGY Phase 3 clinical program. Dr. Mozaffarian is a cardiologist and epidemiologist serving as the Jean Mayer Professor of Nutrition & Medicine, and the Dean of the Friedman School of Nutrition Science & Policy at Tuft’s University. His widely-published research focuses on how diets, such as those rich in OM3s, and lifestyle influence cardiometabolic health and how effective policies can improve health and wellness.
Late in 2017, based on feedback from the FDA, we finalized our Chemistry, Manufacturing, and Controls plans that support our TRILOGY Phase 3 program. The protocol for the TRILOGY 1 and 2 trials had input from and was approved by the FDA, and was essentially of the same standard design as has been used by all other companies having run previous trials in sHTG. In parallel with our Phase 3 clinical trial planning, additional cGMP production lots of our NKPL66 API and CaPre were manufactured, enabling us to build the CaPre and placebo inventory required to support the activated clinical trial sites and complete patient randomization. In the first calendar quarter of 2018, additional RKO was purchased and additional lots of CaPre were manufactured with this material for use in our TRILOGY Phase 3 program. With manufacturing of clinical trial material completed in 2019, our technical resources have been allocated to other activities related to the scale-up of manufacturing for a potential commercial launch of CaPre in early 2022.
| 18 |
Working with a major clinical research organization, we initiated our TRILOGY Phase 3 program and began site activation and patient enrollment at the end of 2017. The TRILOGY studies continued to progress on schedule throughout 2018 and 2019, and by the end of September 2019, both Phase 3 TRILOGY trials had reached 100% patient randomization at clinical sites across the United States, Canada and Mexico. The last visit for the last patient randomized in TRILOGY 1 occurred at the end of November 2019, and the last visit for the last patient randomized in TRILOGY 2 occurred in early January 2020.
The following chart illustrates the design and dosing of our TRILOGY Phase 3 program for CaPre.
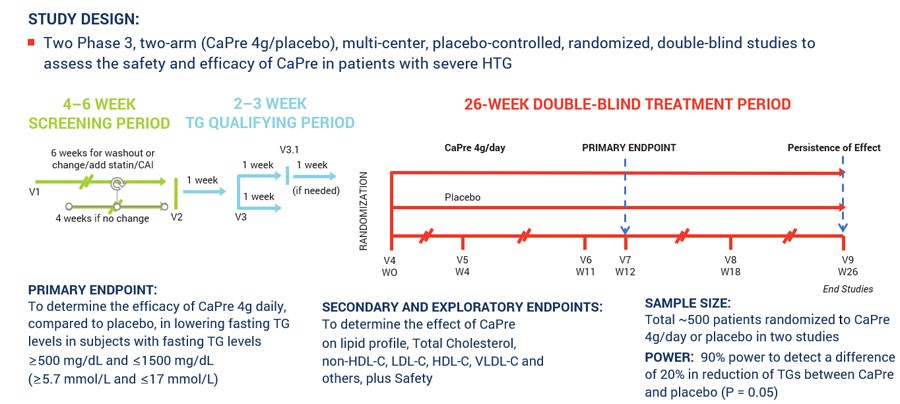
Our first Phase 3 clinical trial, designated as TRILOGY 1, was conducted exclusively in the United States and was fully randomized with a final total of 242 patients. On January 13, 2020, we released topline results for TRILOGY 1, which, despite meaningful TG-lowering in the CaPre arm of the study, did not reach statistical significance due to an unusually large placebo effect described in more detail below. Our second Phase 3 clinical trial, designated TRILOGY 2, which is also fully randomized with a total of 278 patients, is being conducted in the United States, Canada and Mexico, and remains blinded pending proposed modifications to the SAP based on feedback from the FDA. We expect to report TRILOGY 2 topline results by the end of August 2020.
TRILOGY 1 Topline Results
On January 13, 2020, we announced preliminary topline results for the primary endpoint (TG reduction at 12 and 26 weeks) from our Phase 3 TRILOGY 1 trial for CaPre.
We reported a 30.5% median reduction in TG levels among all patients receiving CaPre, compared to a 27.5% median reduction in TG levels among patients receiving placebo at 12 weeks. We also reported a 42.2% median reduction in TGs among patients receiving CaPre while on background statin therapy at 12 weeks, compared to a 31.5% median reduction in TG levels among patients receiving placebo and on background statin therapy. In addition, we reported a 36.7% median reduction in TG levels among patients receiving CaPre at 26 weeks (end of the study), compared to a 28.0% median reduction in TG levels among patients receiving placebo. Both the placebo and CaPre study groups experienced significant reductions in TGs within the first four weeks from baseline, and even though the difference at 12 and 26 weeks was in favor of CaPre, due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance. The safety profile of CaPre in TRILOGY 1 was similar to placebo, as there was no significant difference in treatment-related serious adverse events in the trial. Results for all of the secondary and exploratory endpoints as well as topline results for TRILOGY 2 have subsequently been delayed, pending our investigation into the unusually large placebo effect observed in TRILOGY 1.
| 19 |
The observed reductions in TG levels in the TRILOGY 1 placebo group were far greater than that seen in any previous TG-lowering trial with a prescription OM3. The placebo used in the TRILOGY trials is simple cornstarch, which is a complex carbohydrate with a low glycemic index, and consequently would be expected to have a neutral effect on key biomarkers of patients in the placebo group. In similar previously conducted TG-lowering trials involving prescription OM3 preparations, the placebo responses (using corn oil, olive oil, or vegetable oil) ranged from a change of +16% to -17% across 18 interventions arms, with 14 of 18 arms ranging between +10% to -10%. Note that a low fat diet contains approximately 55% of energy as carbohydrates, and represents approximately 180-220g of carbohydrates per day. Consequently, an additional 4 grams/day of cornstarch (representing roughly 2% of daily intake) would not significantly add to this expected daily intake. In addition, cornstarch is generally regarded as safe (GRAS) and is a commonly used placebo in the pharma industry (the so-called “sugar pill”) that is well known to be an inert and inactive excipient, with low nutritive value. This justification was also noted by FDA.
A table summarizing the placebo and active TG-lowering results from all of these previous HTG trials is presented below:
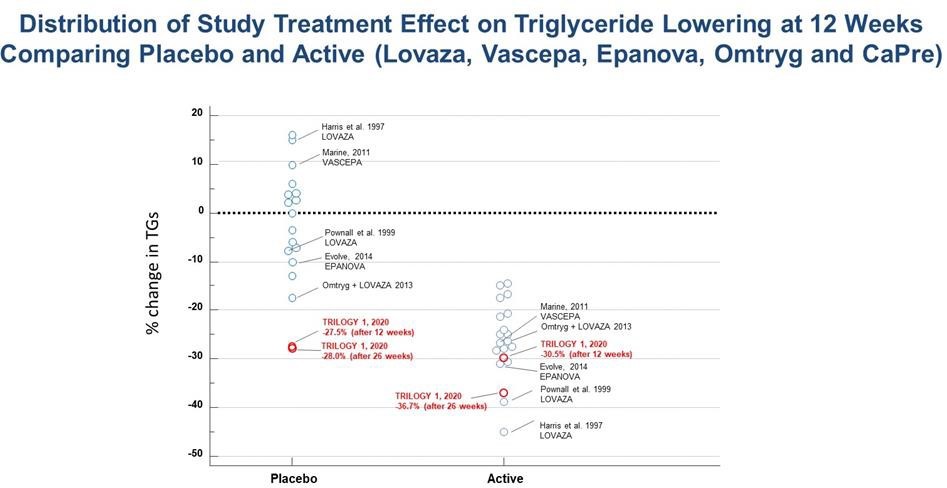
With more investigation, we noted that 5 sites out of the total 54 enrolling sites disproportionately contributed to this placebo response and accounted for approximately 36% of the 242 patients enrolled in the TRILOGY 1 trial. By comparison, the TRILOGY 2 trial was conducted at 71 sites in Canada, Mexico and the United States that enrolled a total of 278 patients. The 5 sites also participated in the TRILOGY 2 trial; however, these sites accounted for only 12% of the total patients, with the majority of these patients coming from only two sites.
Despite monitoring activities conducted throughout the TRILOGY 1 trial to ensure adherence to the protocol and to identify protocol violations, we subsequently identified some unexpected and inconsistent findings that we believed may have negatively contributed to the overall topline results. These findings were explored via a comprehensive and rigorous review of the data and patient medical records, and on site audits of the five sites conducted by an independent team of auditors. To support this effort, we, our independent contract research organization, or CRO, that conducted the TRILOGY trials, our principal investigator Dr. Mozaffarian, and other clinical and regulatory advisors, conducted a thorough review of all data from patients taking both CaPre and placebo. These site audits and the post-hoc investigations of the data were completed in March 2020, and a Type C meeting request was filed with the FDA on April 1, 2020 with the intent to discuss the TRILOGY 1 data and gain alignment with the FDA on the interpretation of the results. We sought the FDA’s input on our proposed revisions to the pre-specified TRILOGY 2 SAP, and a proposal for pooling the data from the TRILOGY 1 and TRILOGY 2 clinical trials in support of an NDA filing. All of the findings and data were summarized and compiled into a briefing package that was filed with the FDA on April 29, 2020.
| 20 |
Given the need to complete the audits and extensive post-hoc review of the TRILOGY 1 data and to obtain FDA feedback, we decided to postpone the unblinding of the topline results for TRILOGY 2 until the third calendar quarter of 2020. Accordingly, key secondary and exploratory endpoints from both TRILOGY 1 and TRILOGY 2 trials are expected as soon as possible after the unblinding of TRILOGY 2 results. We continue to remain blinded to the TRILOGY 2 data, and now that we have feedback from the FDA, we intend to finalize the SAP and submit it to the FDA by the end of July 2020 and expect to report topline results by the end of August 2020.
TRILOGY 1 Findings based on Post-Hoc Analyses and Audits
Following reporting of the TRILOGY 1 topline results in January 2020, we conducted a series of data investigations and analyses, which confirmed no apparent aberration in treatment allocation, capsule contents, mismatched randomization, or systematic errors in the unblinding or final transfer of laboratory data prior to the biostatistical analyses. We confirmed that the CaPre and placebo groups were similar in demographic and baseline characteristics and found no imbalances that could account for the unusually high placebo response. As part of the investigation, we analyzed various other factors between treatment arms, such as washout or discontinuation of lipid-lowering medications at screening, use of lipid-lowering medications at randomization and subsequent change in these medications during the study, use of anti-diabetic medication at randomization, and subsequent change during the study. The protocol for TRILOGY 1 and TRILOGY 2 had input from and was approved by the FDA, and was essentially of the same standard design as has been used by all other companies running trials in drug candidates for the treatment of sHTG. Our protocol required patients to either be washed out or stabilized on any medications that could lower TGs during the four to six week study screening period, before they entered the two to three week qualifying period prior to study randomization. Overall, it was found that subjects in the placebo arm had slightly lower rates of discontinuation (or wash-out) of lipid-lowering medications at screening (about 45% in the placebo group vs 50% in the CaPre group). We also explored in a sub-group analysis the treatment effect of discontinuation of lipid-lowering medications, and it did not reveal any differences. The overall use of lipid-lowering medications was very similar between subjects in the placebo arm (42%) and CaPre arm (45%), and the overall use of anti-diabetic medication was also similar between the two arms (45% in placebo vs 52% in CaPre). It is unlikely that differences in these concomitant medications could explain the placebo response observed in the TRILOGY 1 trial.
A Phenomenon that we Refer to as Triglyceride “Normalization” was Identified between the Qualification and Randomization Periods – Prior to Patients Starting on Drug or Placebo
Subsequent analysis of the TRILOGY 1 clinical data revealed a rapid, significant and sustained reduction in TG levels during the patient qualification period, which took place between screening and the time of patient randomization (that is, prior to patients starting on either drug or placebo). We refer to this phenomenon as “Pre-Randomization TG Normalization”. This phenomenon, which to our knowledge has not been reported in any other TG studies, resulted in an artefactual overestimation of TG reduction in both treatment groups. However, the Pre-Randomization TG Normalization was much greater in the placebo group as compared to the CaPre group, resulting in a significant underestimation of the post-randomization treatment effect of the active drug in TRILOGY 1 and further compromising the ability of the study to detect a clinically significant drug treatment effect.
TG values are normally quite variable, and it is expected that the intra-individual TG variation in subjects on a healthy, low fat National Cholesterol Education Program diet may be 10% or greater (going in either direction) within a one- to two-week period. Thus, it is standard practice to include two or three pre-randomization TG measurements in the determination of the baseline for the calculation of the primary endpoint. The pre-randomization reduction in TGs across all subjects in TRILOGY 1 was approximately 20%, with 25% of all subjects experiencing a reduction equal to or greater than 38%. The median TG normalization reached 30% or more in 12 out of 54 sites (or in 22% of all randomizing sites); in all, much greater than the 10% variation that would have been expected based on physiological variability. In addition, natural variability would have resulted in both increases and decreases in individual levels which would largely offset each other, limiting aggregate variability below 10%. The magnitude of pre-randomization reduction in TG levels seen in TRILOGY 1 indicated a largely unidirectional variability, which was not likely due solely to physiological intra-individual variation, and we therefore consider to be artefactual.
The unexpected and large magnitude of this pre-randomization TG normalization phenomenon resulted in about 40% of all randomized and eligible subjects having TG levels at randomization (Visit 4 or “Week 0”) that fell below the protocol-specified average qualification lower threshold of ≥ 500 mg/dL for patients with sHTG.
| 21 |
Based on the above observations, we believe that the pre-randomization normalization in TG levels substantially impacted the outcome of TRILOGY 1, and the ability of the study to accurately determine the therapeutic impact of CaPre as measured by the pre-specified primary endpoint. Specifically, we believe that the use of an average of 3 values for the calculation of the baseline TG levels corresponding to time points during qualification (e.g., at Week minus 2, and Week minus 1 prior to randomization), and Week 0 (at randomization), resulted in an overestimation of the TG reduction, particularly in the placebo group – with significant TG reduction occurring in many patients even before either drug or placebo were started.
We conducted post-hoc analyses of the primary endpoint using a revised, single point baseline value from Week 0 (Visit 4), the date of randomization, which is referred to as the “Revised Baseline.” Furthermore, only those subjects meeting the protocol-specified TG threshold of ≥ 500 mg/dL and ≤ 1500 mg/dL at Week 0 were included in this post-hoc analyses.
This revised approach for calculating the baseline TG levels corrected for a significant amount of the pre-randomization TG reduction in the subjects that were most affected by this normalization phenomenon. After patients with TG values <500 mg/dL and >1500 mg/dL on the date of randomization were removed, a total of 143 subjects remained (originally N = 242), including 42 subjects in the placebo group (originally N = 69), and 101 subjects remained in the CaPre group (originally N = 173), and were included in the post-hoc analyses, representing 61% and 58% of all randomized subjects, respectively.
In this post-hoc analysis of subjects with TG levels meeting the protocol-specified TG threshold of >500 mg/dL and <1500 mg/dL at Week 0, subjects receiving CaPre showed a 28.1% median reduction in TG levels compared to a 15.4% median reduction among subjects receiving placebo after 12 weeks (this represents the primary endpoint, and a non-adjusted absolute difference of 12.7%; p = 0.29). As compared to the original analysis, the Revised Baseline attenuated the placebo response by approximately 12 percentage points (from -27.5% to -15.4%), while the response in the CaPre arm remained mostly unaffected (reduced from -30.0% to -28.1%). After 26 weeks of double-blind treatment, the efficacy of CaPre showed good persistency of effect with a 32.6% median reduction compared with a 14.6% median reduction in the placebo group, reaching a non-adjusted difference of -18.0%, which trended toward statistical significance (p = 0.0899). As compared to the original analysis, the Revised Baseline attenuated the placebo response at 26 weeks by approximately 13 percentage points (from -28.0% to -14.6%), while the response in the CaPre arm remained mostly unaffected (reduced from -36.7% to -32.6%). The median TG reductions for CaPre as demonstrated using this post-hoc methodology compare favorably to those of previous published studies of other FDA approved drugs for sHTG.
The subgroup of subjects with Revised Baseline TG levels above 750 mg/dL at Visit 4 (Week 0) represented 41% of the subjects retained in the post-hoc analyses. Within this group, the median TG reduction in the subjects receiving CaPre was larger, as would be expected, reaching 36.3% and 43.0% at Week 12 and Week 26, respectively. In comparison, the median TG reduction for the placebo group was 11.8% at Week 12 and 14.4% at Week 26, resulting in non-adjusted differences of 24.5% and 28.6% respectively in favor of CaPre (p = 0.22 and 0.15, respectively).
Not surprisingly, a post-hoc power calculation revealed that these substantially smaller sample sizes resulted in reduced statistical power to detect a treatment difference of 20% as specified in the original SAP. We believe that these post-hoc results suggest clinical relevance even if statistical significance was not demonstrated, as it is plausible that the trend revealed in the post-hoc analysis may have achieved statistical significance with a larger sample size.
| 22 |
Conclusions
In summary, the post-hoc analyses of TRILOGY 1 data using the Week 0 (Visit 4) value as a Revised Baseline in subjects with TG ≥ 500 mg/dL and ≤ 1500 mg/dL at Week 0 showed a strong trend towards correcting for the unexpectedly large placebo response observed in the original analysis, without major changes in the CaPre response observed, and we believe allows for a clearer understanding of the impact on the TG primary endpoint and the potential therapeutic effect of CaPre. However, the median difference in TG levels between CaPre and placebo from the TRILOGY 1 post-hoc analyses still fell short of reaching statistical significance at the Week 12 primary endpoint in this patient-adjusted sample.
Response from the FDA on our Meeting Request and Briefing Package and Next Steps
We provided all of the TRILOGY 1 background information and accompanying data to the FDA in a Type C briefing package filed on April 29, 2020. The FDA provided us with a written response to our Type C Meeting request and briefing package, and confirmed that it will require pivotal efficacy analyses for TRILOGY 2 to be performed on the full Intent to Treat, or ITT, population as contemplated in the original SAP, and the FDA supported the conduct of post-hoc analyses in TRILOGY 1 for exploratory purposes. Consistent with our prior disclosures and depending on the outcome of TRILOGY 2, an additional clinical study may still be needed prior to an NDA submission for CaPre. We and our expert advisors are now carefully considering the FDA’s comments on the TRILOGY 1 data and will conduct further post-hoc analysis based on the FDA’s feedback.
Our Regulatory Strategy for CaPre
Our strategy is to develop CaPre initially for the treatment of sHTG. The TRILOGY Phase 3 program was designed to evaluate the clinical effect of CaPre on TGs, non-HDL-C, LDL-C, and HDL-C levels together with a variety of other cardiometabolic biomarkers in patients with sHTG.
If our TRILOGY Phase 3 program is successful, we intend to pursue a 505(b)(2) regulatory pathway towards an NDA approval in the United States. A 505(b)(2) regulatory pathway is defined in the U.S. Federal Food Drug and Cosmetic Act, or FDCA, as an NDA containing investigations of safety and effectiveness that are being relied upon for approval and were not, in whole, conducted by or for the applicant, and for which the applicant has not obtained a right of reference. 505(b)(2) regulatory pathways differ from a typical NDA because they allow a sponsor to rely, at least in part, on the FDA’s findings of safety and/or effectiveness for a previously- approved drug. We intend to pursue the 505(b)(2) regulatory pathway as a strategy to leverage the large body of safety data for LOVAZA, which could accelerate and streamline the development of CaPre and reduce associated costs and risks. We conducted our two TRILOGY Phase 3 studies to independently assess CaPre’s effectiveness in lowering TGs, and its broader effect in patients with cardiometabolic disease. Consequently, the use of this 505(b)(2) pathway still allows CaPre to retain its New Chemical Entity, or NCE, status due to its novel, patented OM3 free fatty acid/phospholipid ester formulation.
In connection with our intended use of the 505(b)(2) pathway, the FDA supported our proposal to conduct our Bridging Study that compared CaPre (which has an OM3 free fatty acid/phospholipid composition) with LOVAZA (which has an OM3-acid ethyl esters composition) in healthy volunteers. In February 2017, we met with the FDA at an End-of-Phase 2 meeting where our Bridging Study data was discussed. We confirmed with the FDA the 505(b)(2) regulatory approach to use the safety data for LOVAZA and finalized the study design for our TRILOGY Phase 3 program that would be required for NDA approval.
| 23 |
If our primary endpoint of TG lowering shows statistical significance in TRILOGY 2, we plan to continue discussions with the FDA regarding whether pooled results from the primary analysis populations of TRILOGY 1 and 2 can be used to file an NDA. The following development and regulatory timeline assumes that another clinical study would not be required. However, in the event that the FDA should require another study, NDA approval and launch could be delayed by at least 18 to 24 months.
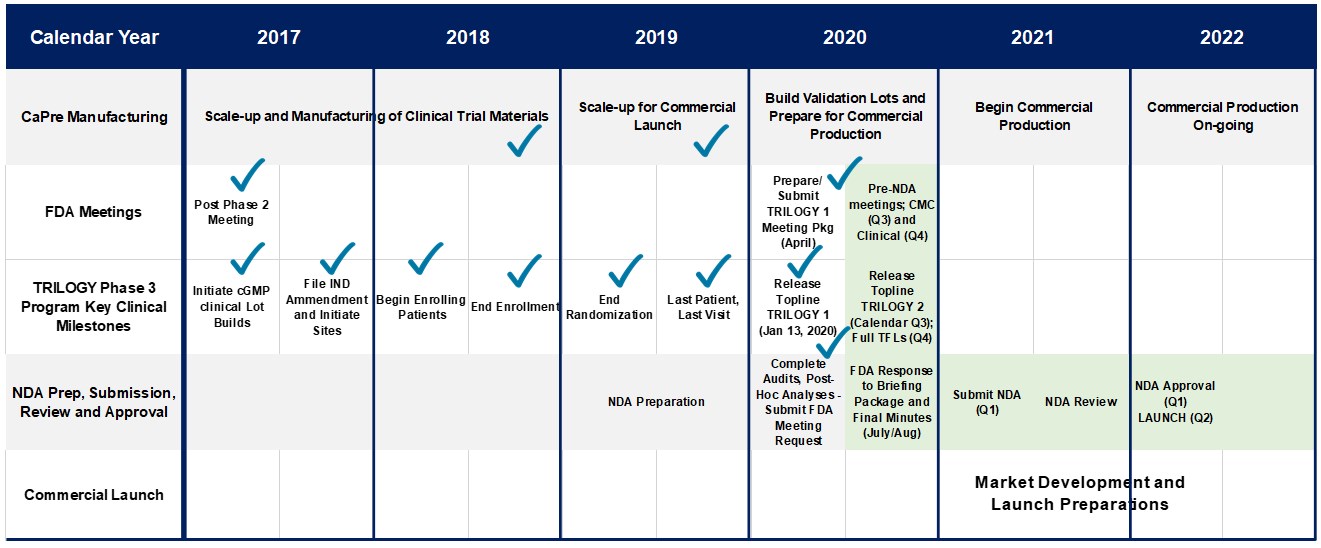
Our Intellectual Property Strategy
Under a license agreement we entered into with Neptune in August 2008, which was later amended on February 9, 2009 and March 7, 2013 (the “License Agreement”), we received an exclusive license to use certain intellectual property of Neptune (which includes several patents) to develop and commercialize CaPre and our novel APIs for use in pharmaceutical and medical food applications in the cardiometabolic field. The term of the License Agreement expires on the date of the last-to-expire licensed patents in 2022. As the result of a royalty prepayment transaction we entered into with Neptune on December 4, 2012, we are not required to pay any royalties to Neptune under the License Agreement during its term for the use of the licensed intellectual property.
Upon the expiry of the License Agreement and related patents, we believe that CaPre will be covered under our own issued and pending patents, and we do not believe that we will afterwards require any licenses to support the commercialization of CaPre.
We currently have patents granted and allowed in the following jurisdictions: United States, Canada, Russia, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, United Kingdom, Italy, Netherlands, Norway, Portugal, Sweden, Japan, Israel, Australia, China, Mexico, Panama, Saudi Arabia, Taiwan, South Africa, Chile, South Korea and Hong Kong. We continue to expand our own intellectual property patent portfolio. We have filed patent applications in more than 20 jurisdictions, including with the European Patent Office (but excluding the individual countries where we have subsequently registered), and in all major countries in North America, Asia and Australia for our “Concentrated Therapeutic Phospholipid Composition”, or proprietary composition, to treat HTG. We currently have more than 20 issued or allowed patents (including in registered European countries) and numerous patent applications pending. A patent is generally valid for 20 years from the date of first filing. However, patent terms can be subject to extensions in some jurisdictions in order to compensate, for example, for delays caused by the patent office during prosecution of the patent application or for regulatory delays during the pre-market approval process.
On January 9, 2019, we announced a Certificate for a European Patent had been issued by the European Patent Office. The granted patent is valid until 2030 and relates to a concentrated phospholipid composition and method of using the same for modulating blood lipids. This patent was validated in Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, United Kingdom, Italy, Netherlands, Norway, Portugal and Sweden.
In May 2019, we announced that we had received notices of allowances for both composition of matter and methods of use patents by the Mexican, Chilean and the Israeli Patent Offices.
In June 2019, we received a notice of allowance for our second patent to be awarded in the People’s Republic of China.
| 24 |
In March 2020, we were awarded a notice of allowance for an additional composition of matter and method of use patent from the U.S. Patent and Trademark Office, our 4th patent to be awarded in the United States, along with a notice of allowance for a composition of matter and method of use patent that was awarded by the Mexican Patent Office, our 3rd patent to be awarded in Mexico. In addition, we also received a favorable decision issued by the Japan Patent Office following an opposition proceeding filed by a third party against our divisional application, confirming its validity. This decision resulted in the allowance of our second major patent in Japan. In April 2020, we received a notice of allowance for our second patent to be awarded by the Canadian Intellectual Property Office and a certificate of patent for our first composition of matter patent to be awarded by the Intellectual Property Office in Hong Kong. These new patents expand our existing claims to include any composition containing EPA and DHA where at least 50% of the composition consists of phospholipids.
In addition to these allowed patents, two Patent Cooperation Treaty, or PCT, applications that cover our encapsulation apparatus and manufacturing process have been filed in all territories under the PCT, while maintaining industrial trade secrets and know-how. The PCT is an international patent law treaty established in 1970. It provides a unified procedure for filing patent applications to protect inventions in each of its contracting states. A patent application filed under the PCT is called an international application, or PCT application. We converted a provisional application covering our starting material composition, known as RKO, for the use of CaPre manufacturing into a PCT application. The corresponding PCT application was filed on February 7, 2020. On January 10, 2020, we filed a provisional application to cover other indications of CaPre in inflammatory-related diseases entitled “Composition that promote pro-resolving mediators”. On April 16, 2020, we filed a provisional application to cover an analytical inline process technology utilizing near-infrared spectroscopy for real-time quality monitoring of OM3 formulations.
We believe all of these patents and patent applications increase potential commercial opportunities for CaPre, including through possible licensing and partnership opportunities. We are committed to building a global portfolio of patents to ensure long-lasting and comprehensive intellectual property protection and to safeguard potentially valuable market expansion opportunities.
Our patent No. 600167 in New Zealand, which is in force until 2030 and relates to a concentrated phospholipid composition comprising 60% or greater PL concentration and method of using the same for treating cardiovascular diseases, has been opposed by BIO-MER Ltd. The evidentiary stage in the New Zealand patent opposition has been completed. We are still waiting for the date of the hearing. In our view, no new prior art has been presented that was not already considered in other jurisdictions, such as in the United States, where our patents are in force.
We received a notice issued from the Japan Patent Office indicating that a third party had filed an opposition against our Japanese Patent No. 6346121. A claim amendment was subsequently filed by us, and we were successful in overcoming the prior art cited in the opposition. Consequently, this opposition was abandoned.
The trademark CaPre® is registered in the United States, Canada, Australia, China, Japan and Europe. We are currently in the process of developing a new brand name and logo for CaPre for launch into the U.S. market. That name, once it is developed, will be trademarked in all of the major jurisdictions around the world.
Our Business and Commercialization Strategy
Key elements of our business and commercialization strategy include initially obtaining regulatory approval for CaPre in the United States for sHTG. We plan to launch CaPre ourselves in the U.S. market, if regulatory approval is obtained. Our preferred strategy outside the United States is to commercialize CaPre through regional or country-specific strategic partnerships, and to potentially seek support and funding from each partner for in-country clinical development, registration and commercialization activities. We believe that a late development-stage and differentiated drug candidate like CaPre could be attractive to various global, regional or specialty pharmaceutical companies, and we are taking a targeted approach to partnering and licensing in various geographies.
Our key commercialization goals include:
| · | complete our TRILOGY Phase 3 program and, assuming the results are positive, file an NDA by early 2021 to obtain regulatory approval for CaPre in the United States, initially for the treatment of sHTG, with the potential to afterwards expand CaPre’s indication to the treatment of high TGs (although at least one additional clinical trial would likely be required to expand CaPre’s indication to this segment); |
| 25 |
| · | continue to strengthen our patent portfolio and other intellectual property rights; |
| · | continue planning for the launch of CaPre in the United States; |
| · | continue to pursue strategic opportunities outside of the United States, such as licensing or similar transactions, joint ventures, partnerships, strategic alliances or alternative financing transactions, to provide development capital, market access and other strategic sources of capital; and | |
| · | continue to search for additional new assets for in-licensing or acquisition that could be synergistic with CaPre, and leverage our commercial organization. |
We expect that additional time and capital will be required to complete the filing of an NDA to obtain FDA approval for CaPre in the United States, and to complete business development collaborations, marketing and other pre-commercialization activities before reaching the commercial launch of CaPre in the United States.
Competition
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to CaPre. We believe that the number of companies seeking to develop products and therapies similar to CaPre will likely increase, particularly based on the positive REDUCE-IT CVOT results by Amarin.
Our competitors in the United States and globally include large, well-established pharmaceutical companies, specialty pharmaceutical sales and marketing companies, and specialized cardiovascular treatment companies. GlaxoSmithKline plc, which currently sells branded LOVAZA, a prescription-only OM3 fatty acid indicated for patients with sHTG, was approved by the FDA in 2004 and has been available in the U.S. market since 2005. Multiple generic versions of LOVAZA are now available in the United States. Amarin launched its prescription-only OM3 drug VASCEPA in 2013, and based on a recent Symphony Health Analytics prescription audit, Amarin had reached approximately 64% market share based on U.S. dollars, and approximately 53% of market share based on units by August 2019. On March 30, 2020, the U.S. District Court for the District of Nevada ruled in favor of two generic companies (Hikma Pharma and Dr. Reddy’s Laboratories) by deciding that Amarin’s patent claims for VASCEPA were found to be invalid for being obvious in view of prior art. Amarin has filed an appeal, and both parties have requested the U.S. Court of Appeals for the Federal Circuit to review Amarin's appeal on an expedited schedule, with a decision expected later this year. Should Amarin lose this appeal, we would expect generic versions of VASCEPA to enter the market within the next year. In addition, EPANOVA (OM3-carboxylic acids) capsules, a free fatty acid form of OM3 (comprised of 55% EPA and 20% DHA), is FDA-approved for patients with sHTG. Omtryg, another OM3-acid fatty acid composition developed by Trygg Pharma AS, received FDA approval for sHTG. Neither EPANOVA nor Omtryg have yet been commercially launched. OMTRYG’s results were inferior to LOVAZA and VASCEPA, and STRENGTH, the long term CVOT trial sponsored by AstraZeneca, was terminated early for reasons that have not yet been reported. Matinas Biopharma recently started their development program for MAT9001, an OM3 free fatty acid that consists primarily of EPA and docosapentaenoic acid. Other large companies with products that would compete indirectly with CaPre include AbbVie, Inc., which currently sells TRICOR and TRILIPIX for the treatment of sHTG, and NIASPAN, which is primarily used to raise HDL-C but is also used to lower TGs. Generic versions of TRICOR, TRILIPIX, and NIASPAN are also now available in the United States. In addition, we are aware of a number of other pharmaceutical companies that are developing non-OM3 products that, if approved and marketed, could compete with CaPre.
Raw Materials
We use semi-refined RKO as our primary raw material to produce CaPre. Krill are generally harvested in Antarctic waters. Krill represent the world’s most abundant biomass, which is monitored by industry regulators to help ensure sustainable cultivation. Historically, we had sourced all of our RKO from Neptune. On August 8, 2017, Neptune announced it was discontinuing krill oil production, and sold its krill oil inventory and intellectual property to Aker. In the three-month period ending December 31, 2017, we purchased a reserve of RKO from Neptune and Aker that was used in the production of CaPre capsules for our TRILOGY Phase 3 clinical program. Additional RKO was purchased from Aker in 2019, which was also used in our TRILOGY Phase 3 program, and will be used to build early commercial inventory. There are several alternative suppliers of RKO that we have confirmed can meet our specifications for CaPre. Combined, they have more than adequate production capacity to meet our future commercial needs.
| 26 |
Employees, Specialized Skills and Knowledge
Our management team consists of professionals from business development, sales and marketing, clinical development, pharmaceutical manufacturing, finance and science backgrounds. Our research team includes scientists with expertise in pharmaceutical development, chemistry, manufacturing and controls, nonclinical and clinical studies, pharmacology, regulatory affairs, quality assurance/quality control, intellectual property and strategic alliances. We currently employ 32 full-time and part-time employees, with the majority working out of our headquarters in Laval Quebec, Canada and at our laboratory in Sherbrooke, Quebec, Canada. We began investing in a commercial leadership team in 2018, and now have 5 senior-level employees based in the United States. We generally require all of our employees to enter into invention assignment, non-disclosure and non-compete agreements. We also rely on third-party consultants and contractors from time to time. Our employees are not covered by any collective bargaining agreement nor represented by a trade union.
Additional Information About Our Phase 2 Clinical Trials
Our COLT Trial
Our COLT clinical trial, which was completed in 2014, was a randomized, open-label, dose-ranging, multi-center trial in Canada designed to assess the safety and efficacy of CaPre in the treatment of patients with TG levels between 200-877 mg/dL. The primary objectives of the COLT study were to evaluate the safety and efficacy of 0.5 gram, 1 gram, 2 grams and 4 grams of CaPre per day in reducing fasting plasma TGs over 4 and 8 weeks, as compared to the standard of care alone.
The secondary objectives of the COLT study were to evaluate:
| · | the effect of CaPre on fasting plasma TGs in patients with TGs between 200-499 mg/dL (mild to moderate HTG); |
| · | the dose dependent effect on fasting plasma TGs in patients with TGs between 500-877 mg/dL (sHTG); and |
| · | the effect of CaPre on fasting plasma levels of LDL-C (direct measurement), HDL-C, non-HDL-C, hs-CRP and OM3 index. |
The final results of the COLT trial indicated that CaPre was safe and effective in reducing TGs in patients with mild to sHTG with significant mean (average) TG reductions above 20% after 8 weeks of treatment with daily doses of 4 grams and 2 grams. Demographics and baseline characteristics of the patient population were balanced in terms of age, race and gender. A total of 288 patients were enrolled and randomized and 270 patients completed the study, which exceeded our targeted number of evaluable patients. From this patient population, approximately 90% had mild to moderate HTG.
The proportion of patients treated with CaPre that experienced one or more adverse events in the COLT trial was similar to that of the standard of care group (30.0% versus 34.5%, respectively). A substantial majority of adverse events were mild (82.3%) and no severe treatment-related adverse effects were reported. Only one patient was discontinued from the study due to an adverse event of moderate intensity. While the rate of gastrointestinal side effects was higher in the CaPre groups compared to standard of care alone and appeared to increase in a dose-related manner, none of the subjects participating in the study suffered from a serious adverse event. The COLT study results showed that even at higher doses, CaPre is safe and well tolerated with only transient and predominantly mild adverse events occurring at low rates.
The COLT trial met its primary objective of showing CaPre to be safe and effective in reducing TGs in patients with mild to sHTG. After only a 4-week treatment, CaPre achieved a statistically significant TG reduction as compared to standard of care alone. Standard of care could be any treatment physicians considered appropriate in a real-life clinical setting and included lifestyle modifications as well as statins and/or ezetimibe. Patients treated with 4 grams of CaPre per day over 4 weeks reached a mean TG decrease of 15.4% from baseline and a mean improvement of 18.0% over the standard of care. Results also showed increased benefits after 8 weeks of treatment, with patients on a daily dose of 4 grams of CaPre registering a mean TG decrease of 21.6% from baseline and a mean improvement of 14.4% over the standard of care.
| 27 |
After 8 weeks of treatment, patients treated with 1 gram of CaPre for the first 4 weeks of treatment and 2 grams for the following 4 weeks, showed a statistically significant TG mean improvement of 16.2% over the standard of care, corresponding to a 23.3% reduction for the 1-2 grams patient population as compared to a 7.1% reduction for the standard of care. After 8 weeks of treatment, patients treated with 2 grams of CaPre for the entire 8 weeks showed statistically significant TG mean improvements of 14.8% over the standard of care, corresponding to a 22.0% reduction for the 2 grams group as compared to a 7.1% reduction for the standard of care. Also, after 8 weeks of treatment, patients treated with 4 grams for the entire 8 weeks showed statistically significant TG, non-HDL-C and HbA1C mean reductions of 14.4% and 9.8% and 15.0%, respectively, as compared to standard of care. The 4-gram group showed mean improvements in:
| · | Improvement of TG levels of 14.4%, corresponds to a reduction of 21.6% as compared to a reduction of a 7.1% for the standard of care group; |
| · | Improvement of non-HDL-C of 9.8%, corresponds to a reduction of 12.0% as compared to a reduction of 2.3% for the standard of care group; and |
| · | Improvements of HbA1C of 15.0%, corresponds to a reduction of 3.5% as compared to an increase of 11.5% for the standard of care group. |
In addition, all combined doses of CaPre showed a statistically significant treatment effect on HDL-C levels, with an increase of 7.4% as compared to standard of care. Trends (p-value < 0.1) were also noted on patients treated with 4 grams of CaPre for the entire 8-week treatment period with mean reduction of total cholesterol of 7.0% and increase of HDL-C levels of 7.7%, as compared to the standard of care. The results of the COLT trial indicated that CaPre has no significant deleterious effect on LDL-C levels.
Our TRIFECTA Trial
Our TRIFECTA clinical trial, which was completed in 2015, was a 12-week, randomized, placebo-controlled, double-blind, dose-ranging trial in Canada, designed to assess the safety and efficacy of CaPre at a dose of 1 gram or 2 grams on fasting plasma TGs as compared to a placebo in patients with TG levels between 200-877 mg/dL. A total of 387 patients were randomized and 365 patients completed the 12-week study, consistent with our targeted number of evaluable patients. From this patient population, approximately 90% had mild to moderate HTG with baseline TGs between 200 and 499 mg/dL. The remainder had sHTG with baseline TGs between 500 and 877 mg/dL. Approximately 30% of patients were on lipid-lowering medications, such as statins, and approximately 10% were diabetic.
Similar to our COLT study, the primary objective of the TRIFECTA study was to evaluate the effect of CaPre on fasting plasma TGs in patients with TGs between 200-877 mg/dL and to assess the tolerability and safety of CaPre. The secondary objectives of the TRIFECTA study were to evaluate:
| · | the effect of CaPre on fasting plasma TGs in patients with TGs between 200-499 mg/dL; |
| · | the dose dependent effect on fasting plasma TGs in patients with TGs between 500-877 mg/dL; and |
| · | the effect of CaPre in patients with mild to moderate HTG and sHTG on fasting plasma levels of LDL-C (direct measurement), and on fasting plasma levels of HDL-C, non-HDL-C, hs-CRP and OM3 index. |
| 28 |
CaPre successfully met the TRIFECTA study’s primary objective. The placebo-corrected percentage change in TGs were decreases of 9.1% (p=0.049) and 9.7% (p=0.044) for 1 gram and 2 grams of CaPre, respectively. Key secondary objectives were also met:
| · | there was a statistically significant decrease in non-HDL-C versus placebo (p=0.038), with the 2-gram group decreasing by 5.3% from baseline versus placebo over the 12-week period; |
| · | HDL-C slightly increased at both the 1-gram and 2-gram levels; and |
| · | LDL-C and slightly decreased at the 2-gram level. |
Finally, a statistically significant dose response increase in the OM3 index for patients on 1 gram and 2 grams versus placebo was noted. The OM3 index reflects the percentage of EPA and DHA in red blood cell fatty acids and the risk of cardiovascular disease is considered to be lower as the OM3 index increases.
CaPre was found to be safe and well tolerated at all doses tested, with no serious adverse events that were considered treatment- related. Out of 387 randomized patients, a total of 7 (1.8%) were discontinued as a result of adverse events, three were on placebo, two were on 1 gram and two were on 2 grams of CaPre. The predominant incidence was gastrointestinal-related, with no difference between CaPre and placebo. The safety profiles of patients on CaPre and placebo were similar.
Government Regulation
United States Drug Development
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record- keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug products such as CaPre. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific to each regulatory authority, submitted for review and approved by the regulatory authority.
FDA Regulatory Process
In the United States, the FDA regulates drugs under the FDCA, and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state and local statutes and regulations require the expenditure of substantial time and financial resources.
In order to be marketed in the United States, CaPre must be approved by the FDA through the NDA review process. The process required before a drug may be marketed in the United States generally involves the following:
| · | completion of extensive nonclinical (animal) and formulation studies in accordance with applicable regulations, including the FDA’s GLP regulations; |
| · | submission of an investigational new drug application, or IND, which must become effective before human clinical trials may begin in the United States; |
| · | performance of adequate and well-controlled clinical trials in accordance with the applicable IND and other clinical study- related regulations, such as current Good Clinical Practices, or cGCP, to establish the safety and efficacy of the proposed drug for its proposed indication; |
| · | submission of an NDA for a new drug; |
| · | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| 29 |
| · | satisfactory completion of potential FDA audit of the nonclinical and/or clinical trial sites that generated the data in support of the NDA; and |
| · | FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States. |
The data required to support an NDA is generated in two distinct development stages: nonclinical and clinical. The nonclinical development stage generally involves synthesizing or otherwise producing the active component, developing the formulation and determining the manufacturing process, as well as carrying out non-human toxicology, pharmacology and drug metabolism studies in the laboratory, which support subsequent clinical testing. The sponsor must submit the results of the nonclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND, which is a request for authorization from the FDA to administer an investigational drug product to humans. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials. The FDA may also place the IND on clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. A clinical hold may be imposed at any time before or during a clinical trial due to safety concerns or non-compliance.
The clinical stage of development first involves the administration of the investigational drug to healthy volunteers and then to patients with the disease being targeted with the drug, all done under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with cGCP. All research subjects must provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, data collection, and the parameters to be used to monitor subject safety and assess the investigational drug’s efficacy. Each protocol, and any subsequent amendments to the protocol or new investigator’s information, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or its legal representative. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries, as well as reporting of safety information under the IND.
Clinical studies are generally conducted in three sequential phases that may overlap, known as Phase 1, Phase 2 and Phase 3 clinical trials. Phase 1 generally involves a small number of healthy volunteers who are initially exposed to a single dose and then multiple doses of the investigational drug. The primary purpose of these studies is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the drug. Phase 2 trials typically involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, as well as identification of possible adverse effects and safety risks and preliminary evaluation of efficacy. Phase 3 clinical trials generally involve large numbers of patients at multiple sites, often in multiple countries (from several hundred to several thousand subjects) and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use, and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. Phase 3 clinical trials should, if possible, include comparisons with placebo and may include a comparison to approved therapies. The duration of treatment is often extended to mimic the actual use of a product during marketing. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA (Pivotal Studies).
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA. In addition, written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk.
Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides oversight and will determine whether or not a trial may move forward at designated check points based on review of interim data from the study. A clinical trial may be terminated or suspended based on evolving business objectives and/or competitive climate.
| 30 |
The manufacturing process must be capable of consistently producing quality batches of the investigational drug and, among other things, must develop methods for testing the identity, strength, quality and purity of the final drug product. The sponsor must develop appropriate labeling that sets forth the conditions of intended use. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
Post-approval studies, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 studies as part of a post-approval commitment, such as pediatric studies.
NDA and FDA Review Process
Nonclinical and clinical information is filed with the FDA in an NDA along with proposed labeling. The NDA is a request for approval to market the drug and must contain proof of safety, purity, potency and efficacy, which is demonstrated by extensive nonclinical and clinical testing. Data may come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA.
The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. FDA approval of an NDA must be obtained before marketing a drug in the United States. In addition, under the Pediatric Research Equity Act, an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA has ten months from the filing date in which to complete its initial review of a standard NDA and respond to the applicant. This review typically takes 12 months from the date the NDA is submitted to the FDA including the screening which takes a period of 60 days. The FDA does not always meet its PDUFA goal dates for standard NDAs, and the review process may be significantly extended by FDA requests for additional information or clarification.
After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMPs to assure and preserve the product’s identity, strength, quality and purity. The FDA will likely re-analyze the clinical trial data, which could result in extensive discussions with the FDA.
Before approving an NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether they comply with cGMPs. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. In addition, before approving an NDA, the FDA may also audit data from clinical trials to ensure compliance with cGCP requirements. After the FDA evaluates the application, manufacturing process and manufacturing facilities, it will issue a Complete Response Letter, or CRL. A CRL indicates that the review cycle of the application is complete and whether the application is approved and, when applicable, the CRL describes the specific deficiencies in the NDA and may require additional clinical data and/or an additional Phase 3 clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. The applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
| 31 |
If a product receives marketing approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling, may condition the approval of the NDA on other changes to the proposed labeling, or may require a Risk Evaluation and Mitigation Strategy (REMS), which could limit the ability to market the drug once approved. The FDA may also require the development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and surveillance to monitor the effects of approved products.
U.S. Post-Marketing Requirements
Following approval of a new product, a pharmaceutical company and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and recordkeeping activities, reporting to the applicable regulatory authorities of adverse experiences with the product and reporting Field Alert information relating to bacteriological contamination, significant deterioration of the product or failure of distributed product to meet specifications, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling, or “off-label use”, limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available drugs for off-label uses, manufacturers and distributors may not market or promote such off-label uses. Modifications or enhancements to the product or its labeling or changes of the site of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process. In some cases, these changes will require the submission of clinical data and the payment of a user fee.
U.S. Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of our prescription drug candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing and review of the relevant NDA.
Non-U.S. Drug Regulation
In Canada, biopharmaceutical product candidates are regulated by the Food and Drugs Act and the related rules and regulations, which are enforced by the Therapeutic Products Directorate of Health Canada. In order to obtain approval for commercializing new drugs in Canada, the sponsor must satisfy many regulatory conditions. The sponsor must first complete preclinical studies in order to file a clinical trial application, or CTA, in Canada. The sponsor will then receive different clearance authorizations to proceed with Phase 1 clinical trials, which can then lead to Phase 2 and Phase 3 clinical trials. Once all three phases of trials are completed, the sponsor must file a registration file named a new drug submission, or NDS, in Canada. If the NDS demonstrates that the product was developed in accordance with the regulatory authorities’ rules, regulations and guidelines and demonstrates favorable safety and efficacy and receives a favorable risk/benefit analysis, then the regulatory authorities issue a notice of compliance, which allows the sponsor to market the product.
| 32 |
In addition to regulations in the United States and Canada, we are subject to a variety of regulations governing clinical studies and commercial sales and distribution of our products in other jurisdictions around the world. These laws and regulations typically require the licensing of manufacturing and contract research facilities, carefully controlled research and testing of product candidates and governmental review and approval of results prior to marketing therapeutic product candidates. Additionally, they require adherence to the FDA’s GLP, good clinical practices and good manufacturing practices during production. The process of new drug approvals by regulators in the United States, Canada and the European Union are generally considered to be among the most rigorous in the world.
Whether or not FDA or Health Canada approval is obtained for a product, we must obtain approval from the comparable regulatory authorities of other countries before we can commence clinical studies or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for the FDA or Health Canada approval. The requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement vary greatly from country to country. In some international markets, additional clinical trials may be required prior to the filing or approval of marketing applications within the country.
Active Pharmaceutical Ingredient Regulation
The FDA will regulate finished products containing APIs developed or under development by us. Depending on its intended uses, a finished product containing the API may be regulated as a drug under the procedures described above. In general, the regulatory requirements in other countries also depend on the nature of the finished product and do not focus on the API itself.
Fiscal Year 2020 Developments
| · | On April 1, 2019, we announced publication of CaPre’s bioavailability study in a leading peer-reviewed journal. This study further validated our prior study results demonstrating that the bioavailability of CaPre is significantly better than LOVAZA when taken with a low-fat meal. |
| · | On June 4, 2019, we announced that our TRILOGY 2 clinical trial had achieved 100% randomization, and that more than 60% of patients who had previously been randomized in our Phase 3 TRILOGY trials had completed their 6-month treatment plans. |
| · | On September 9, 2019, we announced that we were awarded up to $750,000 in non-dilutive and nonrepayable funding, as well as technical and business advisory services, from the National Research Council of Canada Industrial Research Assistance Program to apply towards eligible research and development disbursements for our commercial production platform for CaPre. |
| · | On September 30, 2019, we announced that 100% of the required total patients for our two TRILOGY Phase 3 clinical trials had been randomized, and nearly 80% of the patients in both trials combined had completed their 6-month plans. |
| · | On September 30, 2019, we determined that we would migrate from reporting in IFRS to U.S. GAAP effective beginning with this annual report in connection with becoming a U.S. domestic registrant. |
| · | On November 4, 2019, we announced that we had partnered with Aker to deliver to us RKO under a two-year, fixed price supply agreement. |
| · | On November 7, 2019, we announced the publication of a CaPre pharmacokinetics study entitled, “Evaluation of OM3-PL/FFA Pharmacokinetics After Single and Multiple Oral Doses in Healthy Volunteers” in a leading peer-reviewed journal, Clinical Therapeutics. The study showed that the bioavailability of CaPre did not appear to be meaningfully affected by the fat content of a meal consumed before dose administration. |
| · | On November 18, 2019, we released preliminary new animal study data which provided additional insights into CaPre’s potential mechanism of action in diabetes. The preliminary findings obtained for the diabetes mouse study showed that CaPre may promote insulin secretion as seen by statistically significant results produced in a standard glucose challenge test, thus suggesting a mechanism of action different and unique when compared to metformin, which does not promote insulin secretion. |
| 33 |
| · | On November 26, 2019, we announced that the last patient completed their final visit in our TRILOGY 1 Phase 3 trial of CaPre. |
| · | On December 18, 2019, we incorporated a new wholly-owned subsidiary named Acasti Innovation AG under the laws of Switzerland for the purpose of future development of our intellectual property and global distribution of our products. |
| · | On December 23, 2019, we provided an update on the expected delay into January 2020 of topline results for our TRILOGY 1 Phase 3 trial of CaPre. The reporting of TRILOGY 1 was postponed due to an unexpected delay in data processing and transfer from the central testing laboratory to the statistical consultants for independent and external validation. When this problem was identified by the CRO data management group, it triggered an immediate hold on the data transfer to the CRO statistical group and initiated a full quality review by the CRO of the processes and procedures involved at the central testing laboratory. This review was completed in early January 2020, and topline results for TRILOGY 1 were subsequently released on January 13, 2020. A more comprehensive audit of the central laboratory was subsequently completed in the first calendar quarter of 2020. |
| · | On January 9, 2020, we announced that the last patient completed their final visit in our TRILOGY 2 Phase 3 trial of CaPre. |
| · | On January 13, 2020, we reported topline results for our TRILOGY 1 Phase 3 trial of CaPre, which, despite showing a meaningful reduction of TGs in the CaPre arm, did not reach statistical significance due to an unusually large placebo effect. |
| · | On February 10, 2020, we provided an update on our TRILOGY 1 and TRILOGY 2 Phase 3 trials of CaPre. We disclosed that detailed examination of the TRILOGY 1 Phase 3 trial results for CaPre was underway, including specific clinical site audits and an audit of the central testing laboratory. We also announced that once the full analysis of TRILOGY 1 is completed, we intended to request a meeting with the FDA to discuss the data and seek guidance on how to modify the SAP for our TRILOGY 2 trial before unblinding the TRILOGY 2 trial results. |
| · | On March 11, 2020, we announced that a notice of allowance for new composition of matter and method of use patents had been received from the U.S. and Mexican patent offices. |
| · | On April 1, 2020, we announced that a Type C meeting request had been submitted to the FDA, with a meeting expected in the second half of June 2020. |
| · | On April 1, 2020, we also announced the annual grant of stock options to employees, executives and directors as part of our annual performance review in accordance with our Long Term Incentive Plan. |
| · | On April 30, 2020, we announced that we had submitted a briefing package to the FDA on April 29, 2020 for its review. |
| · | On June 19, 2020, we announced that the FDA had provided us with a written response to our Type C Meeting request and briefing package. |
Corporate Structure
Acasti was incorporated on February 1, 2002 under Part 1A of the Companies Act (Québec) under the name “9113-0310 Québec Inc.” On February 14, 2011, the Business Corporations Act (Québec), or QBCA, came into effect and replaced the Companies Act (Québec). We are now governed by the QBCA. On August 7, 2008, pursuant to a Certificate of Amendment, we changed our name to “Acasti Pharma Inc.”, our share capital description, the provisions regarding the restriction on securities transfers and our borrowing powers. On November 7, 2008, pursuant to a Certificate of Amendment, we changed the provisions regarding our borrowing powers. We became a reporting issuer in the Province of Québec on November 17, 2008. On December 18, 2019, we incorporated a new wholly-owned subsidiary named Acasti Innovation AG, or AIAG, under the laws of Switzerland for the purpose of future development of our intellectual property and for global distribution of our products. AIAG currently does not have any operations.
| 34 |
Available Information
This annual report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K, and any amendments to these reports are filed, or will be filed, as applicable, with the Securities and Exchange Commission, or SEC, and the Canadian Securities Administrators, or CSA. These reports are available free of charge on our website, www.acastipharma.com, as soon as reasonably practicable after we electronically file such reports with or furnish such reports to the SEC and the CSA. Information contained on, or accessible through, our website is not a part of this annual report, and the inclusion of our website address in this document is an inactive textual reference.
Additionally, our filings with the SEC may be accessed through the SEC’s website at www.sec.gov and our filings with the CSA may be accessed through the CSA’s System for Electronic Document Analysis and Retrieval at www.sedar.com.
| Item 1A. | Risk Factors |
Investing in our securities involves a high degree of risk due to, among other things, the nature of our business and the present stage of our development. Prospective and current investors should carefully consider the following risks and uncertainties, together with all other information in this annual report, as well as our financial statements included in this annual report and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.” If any of these risks actually occur, our business, financial condition, prospects, results of operations or cash flow could be materially and adversely affected and you could lose all or a part of the value of your investment. Additional risks or uncertainties not currently known to us, or that we deem immaterial, may also negatively affect our business operations.
General Risks Related to the Company
Our business and operations may be materially and adversely affected by the recent COVID-19 pandemic.
The COVID-19 pandemic is severely adversely affecting the U.S., Canadian and many other global economies. If the outbreak continues to spread, it may affect our operations and those of third parties upon which we rely, including:
| · | causing disruptions in the supply chain for our CaPre drug product candidate delaying the scale-up of our manufacturing of CaPre in anticipation of a commercial launch; |
| · | delaying the conclusion of our TRILOGY Phase 3 program due to limited access to expert consultants; |
| · | delaying necessary interactions with regulators (including the FDA) due to limitations in employee resources or furlough of government or contractor personnel; |
| · | limiting our ability to secure funding for continued development and commercial preparations for launch; |
| · | delaying the development and commercial launch of CaPre; |
| · | disrupting the commercialization of CaPre, if and once launched; |
| · | limiting our outreach to physicians so they can be more likely to prescribe CaPre; and |
| · | limiting our ability to recruit professional staff to support the development, launch and commercialization of CaPre. |
The extent to which the COVID-19 pandemic impacts our business and prospects will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the COVID-19 pandemic and the actions to contain the COVID-19 pandemic or treat its impact, among others.
Additionally, while the potential economic impact brought by, and the duration of, the COVID-19 pandemic is difficult to assess or predict, the impact of the COVID-19 pandemic on the global financial markets may reduce our ability to access capital, which could negatively impact our short-term and long-term liquidity and adversely affect our business and overall financial condition.
| 35 |
There is substantial doubt about our ability to continue as a going concern.
We have incurred operating losses and negative cash flows from operations since our inception. To date, we have financed our operations through public offerings and private placements of securities, proceeds from exercises of warrants, rights and options, and receipt of research tax credits and research grant programs. .
Our current assets of $16.1 million as at March 31, 2020 include cash and cash equivalents totaling $14.2 million. Assuming positive results from TRILOGY Phase 3 program, we expect that additional time and capital will be required by us to file an NDA to obtain FDA approval for CaPre in the United States, to further scale-up our manufacturing capabilities, and to complete marketing and other pre-commercialization activities. Consequently, we expect to require additional capital to fund our daily operating needs beyond January 2021. Based on a conservative estimate, we believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements into the first calendar quarter of 2021. To fully execute our business plan, we plan to raise the necessary capital primarily through additional securities offerings as well as non-dilutive sources of capital such as grants or loans and strategic alliances. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay the commercial launch of CaPre, if it receives regulatory approval. Unexpected negative results in our TRILOGY Phase 3 program for CaPre may affect our ability to raise additional capital and/or complete strategic development and/or distribution partnerships to support the commercial launch of CaPre. Additional funding from third parties may not be available on acceptable terms or at all to enable us to continue with the commercialization of CaPre.
If we do not raise additional funds or find one or more strategic partners, we may not be able to realize our assets and discharge our liabilities in the normal course of business. As a result, there is a substantial doubt about our ability to continue as a going concern. Our financial statements have been prepared on a going-concern basis, which assumes we will continue our operations in the foreseeable future, and will be able to realize our assets and discharge our liabilities and commitments in the ordinary course of business. If we are unable to continue as a going concern, material impairments of the carrying value of our assets, including intangible assets, could be required. If we fail to obtain additional financing, we may not be able to continue as a going concern.
We may never become profitable or be able to sustain profitability.
We are a clinical-stage biopharmaceutical company with a limited operating history. The likelihood of the success of our business plan must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered when developing and expanding early-stage businesses and the regulatory and competitive environment in which we operate. Biopharmaceutical product development is a highly speculative undertaking, involves a substantial degree of risk, and is a capital- intensive business. We expect to incur expenses without any meaningful corresponding revenues unless and until we are able to obtain regulatory approval for and can begin selling CaPre in significant quantities. We filed our IND for CaPre in late 2013, which allowed us to initiate clinical development in the United States towards FDA approval for CaPre. To date, we have not generated any revenue from CaPre, and we may never be able to obtain regulatory approval for marketing CaPre in any indication. Even if we are able to commercialize CaPre, we may still not generate significant revenues or achieve profitability. Additionally, we may not be able to attain our targeted cost of goods sold, and levels of insurance reimbursement for CaPre may not be commercially viable in all global markets. We incurred net losses for the fiscal year ended March 31, 2020 of $26.3 million and $39.3 million for the fiscal year ended March 31, 2019. As of March 31, 2020, we had an accumulated deficit of $129.4 million.
We expect that our expenses will increase in the future as we prepare to seek FDA approval for the commercial launch of CaPre.
Our research and development expenses could increase in the future if we decide to develop CaPre for other indications. As a result, we expect to continue to incur substantial losses for the foreseeable future, and those losses may be increasing. We are uncertain about when or if we will be able to achieve or sustain profitability. If we fail to become and remain profitable, our ability to sustain our operations and to raise capital could be impaired and the price of our common shares could decline.
| 36 |
Given the unusually large placebo effect observed in the TG topline results of our TRILOGY 1 Phase 3 clinical trial and that the data for TRILOGY 2 is still blinded, the outcome of our TRILOGY Phase 3 program and our ability to file an NDA in early 2021 remains uncertain.
On January 13, 2020, we released topline results for our TRILOGY 1 trial, which did not reach statistical significance due to an unusually large placebo effect described in more detail in “Item 1. Business — TRILOGY 1 Topline Results”. Our investigation of the underlying data identified some unexpected and inconsistent findings that we believe, based on our audits and subsequent post-hoc data analyses, may have negatively contributed to the unusually large placebo effect. We summarized and provided this information in the form of a briefing package to the FDA, to gain alignment with the FDA on the interpretation of the TRILOGY 1 results and implications for our TRILOGY 2 trial as well as receive the FDA’s inputs on our proposed revisions to the pre-specified TRILOGY 2 SAP.
As we disclosed on June 19, 2020, the FDA provided us with a written response to our Type C Meeting request and briefing package. The FDA confirmed that it will require pivotal efficacy analyses for TRILOGY 2 to be performed on the full ITT population as contemplated in the original SAP and it supported the conduct of post-hoc analyses in TRILOGY 1 for exploratory purposes. Consistent with our prior disclosures and depending on the outcome of TRILOGY 2, an additional clinical study may still be needed prior to NDA submission. Based on the written feedback received from the FDA, we will now finalize the SAP for TRILOGY 2, which we plan to submit to the FDA by the end of July 2020. See “Item 1. Business — Recent Developments”.
There can be no assurance that (i) the FDA will agree with our observations on the TRILOGY 1 data, (ii) we will achieve our primary endpoint or any of our secondary and exploratory endpoints for TRILOGY 2, or (iii) we will be able to report these topline results on a timely basis. The FDA may also not allow us to pool data from TRILOGY 1 and TRILOGY 2 even if we achieve the primary endpoint for TRILOGY 2. The results of pooling the TRILOGY 1 and TRILOGY 2 data and results may not achieve statistical significance or allow for a filing of an NDA. A failure to achieve the primary endpoint for TRILOGY 2 or achieve statistical significance based on the pooling the TRILOGY 1 and TRILOGY 2 data and results could result in the need to repeat one or both TRILOGY trials, which could prevent or delay our NDA submission relating to, or the development and commercialization of, CaPre and have a material adverse effect on our business and financial condition.
If outcome studies being conducted by our competitors testing the impact of OM3 on treating patients with high TGs are negative, there could also be an adverse impact for CaPre.
Top-line results from the cardiovascular outcomes trial, or CVOT, sponsored by Amarin (the REDUCE-IT trial) were released in September 2018. This study was successful, and showed that long-term use of an OM3 therapeutic (VASCEPA) in patients with elevated TGs (>150 mg/dL), resulted in a significant reduction in cardiovascular risk. A second CVOT sponsored by AstraZeneca (the STRENGTH trial) was discontinued on January 13, 2020 due to its low likelihood of demonstrating a benefit to patients with elevated TGs. The potential impacts of the discontinuance of the STRENGTH trial on our business and the OM3 drug market in general are not yet known. Given that the REDUCE-IT trial showed that an OM3 therapeutic drug can effectively treat patients with high TGs and improve cardiovascular, morbidity and mortality outcomes, we believe that the potential exists to expand CaPre’s indication in the future to include the treatment of high TGs (150 – 500 mg/dL); however, this expansion would require at least one additional clinical study, likely a CVOT trial. As a result of the discontinuance of AstraZeneca’s STRENGTH trial, our potential target market for CaPre may be limited to patients with sHTG (for which the total U.S. market was estimated, based on audited prescription data by Symphony Health Analytics, to be approximately $1.65 billion in 2019), and our ability to realize greater market potential for CaPre may be harmed.
We rely on third parties to conduct our TRILOGY Phase 3 program for CaPre.
We rely on CROs to monitor and manage data for our TRILOGY Phase 3 program for CaPre. While we will only control certain aspects of the CRO’s activities, we nevertheless are responsible for ensuring that our clinical trials are conducted in accordance with applicable protocols, and legal, regulatory and scientific standards, and our reliance on the CRO does not relieve us from those responsibilities. We and the CRO are required to comply with current good clinical practices, or cGCPs, which are regulations and guidelines enforced by the FDA, Health Canada and comparable foreign regulatory authorities for any products in clinical development.
The FDA enforces these cGCP regulations through periodic inspections of trial sponsors, principal investigators and trial sites. If we or the CRO fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, Health Canada or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications for CaPre. Upon inspection, the FDA could determine that our clinical trials do not comply with cGCPs. In addition, our clinical trials must be conducted with products produced under cGMP regulations and require a large number of test subjects. If we or the CRO fail to comply with these regulations, we may have to repeat preclinical studies or clinical trials for CaPre, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
| 37 |
If our relationship with a CRO terminates, we may not be able to enter into arrangements with alternative CROs. If the CRO does not successfully carry out its duties or obligations or meet expected deadlines, if it needs to be replaced or if the quality or accuracy of the clinical data it obtains is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, we may have to extend, delay or terminate our preclinical or clinical trials, and we may not be able to obtain regulatory approval for or successfully commercialize CaPre.
The third parties that are conducting our TRILOGY Phase 3 program for CaPre are not our employees and, except for remedies available to us under our agreements with the CROs, we cannot control whether or not they devote sufficient time and resources to our preclinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could affect their performance on our behalf.
We rely on third parties to manufacture, produce and supply CaPre and we may be adversely affected if those third parties are unable or unwilling to fulfill their obligations, including complying with FDA requirements.
Producing pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Currently, while we do own our manufacturing and encapsulation equipment, we outsource the production of CaPre, and do not own or operate the manufacturing facilities. Accordingly, we need to rely on one or more third party contract manufacturers to produce and supply our required drug product for our nonclinical research and clinical trials, and to build commercial inventory for CaPre.
Scale up of our commercial manufacturing processes for CaPre is a difficult and uncertain task, and there are risks associated with scaling to the level required for full commercialization, including, among others, pricing, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability and consistent pricing of reagents or raw materials. Consequently, we may not be able to attain our targeted cost of goods sold for CaPre. Any of these challenges could delay a commercial launch of CaPre, require bridging studies or the repetition of studies or trials, increase development costs, delay approval of CaPre, impair our commercialization efforts, and increase our cost of goods. We may have to delay or suspend the production of CaPre if a third-party manufacturer:
| · | becomes unavailable for any reason, including as a result of the failure to comply with cGMP regulations; |
| · | experiences manufacturing problems or other operational failures, such as equipment failures or unplanned facility shutdowns required to comply with cGMP or damage from any event, including fire, flood, earthquake, pandemics such as an extension of the current COVID-19 pandemic, business restructuring or insolvency; or |
| · | fails or refuses to perform its contractual obligations under its agreement with us, such as failing or refusing to deliver the quantities of CaPre requested by us on a timely basis. |
If our third-party contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with cGMP regulations, we may be subject to sanctions, including fines, product recalls or seizures, injunctions, delays or suspensions of our clinical trials for CaPre, total or partial suspension of production of CaPre, civil penalties, withdrawals of previously granted regulatory approvals, and criminal prosecution. While we contemplate procuring it in the future, we do not currently have arrangements in place for redundant supply. If any one of our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are several potential alternative contract manufacturers who could manufacture CaPre, we may incur added costs and delays in identifying and qualifying any such replacement.
| 38 |
We have historically had no marketing, market access, and sales organization, and as a company, have not previously marketed any new drug products. If we are unable to properly establish marketing, market access, and sales capabilities or enter into agreements with a strategic partner to market and sell CaPre in any key market, we may not be able to generate revenue.
We have historically had no sales, marketing, market access, or distribution capabilities, and as a company, we have also historically not launched any new drug products. If CaPre or another of our future product candidates is approved for commercialization, we plan to develop in-house sales, marketing, market access and sales force capability, which would require significant capital expenditures, management resources and time, unless we can find a strategic partner to assist us with sales, marketing, market access, and distribution. Also, we would have to compete with other biotechnology and pharmaceutical companies to recruit, hire, train and retain marketing and sales personnel. We face competition in our search for strategic partners to assist us with sales, marketing, market access and distribution, and we may not be able to establish or maintain any such arrangements in any key market on terms acceptable to us or at all. If we do find a strategic partner, any revenue we receive from CaPre would partly depend upon the efforts of that strategic partner, which may not be successful. We may have little or no control over the marketing, market access and sales efforts by any strategic partner we find for CaPre and our revenue may be lower than if we had commercialized CaPre independently.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive pharmaceuticals industry largely depends upon our ability to attract and retain highly qualified managerial, scientific, medical, and commercial personnel. Competition for skilled personnel in our market is intense and competition may limit our ability to hire and retain highly qualified personnel on acceptable terms. We are highly dependent on our management, financial, commercial, and scientific personnel. Despite our efforts to retain valuable employees, members of our management, financial, commercial, scientific and medical teams may terminate their employment with us on short notice or, potentially, without any notice at all. The loss of the services of any of our executive officers or other key employees could potentially harm our business, operating results or financial condition. Our success may also depend on our ability to attract, retain and motivate highly skilled junior, mid-level, and senior managers and scientific personnel. In addition, we do not maintain “key person” insurance policies on the lives of our executives or those of any of our other employees. Other pharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do. They also may provide more diverse opportunities, more lucrative compensation packages, and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we can offer. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can develop and commercialize CaPre and any other future product candidates would be limited.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our suppliers, third party manufacturers and other contractors and consultants could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical pandemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to manufacture CaPre. Our ability to obtain supplies of CaPre could be disrupted if the operations of our manufacturers and suppliers are affected by a man-made or natural disaster or other business interruption.
Our prospects currently depend entirely on the success of CaPre, which is still in late stage clinical development, and we may not be able to generate revenues from CaPre.
We have no prescription drug products that have been approved by the FDA, Health Canada or any similar regulatory authority. Currently, our only prescription drug candidate is CaPre, for which we have not yet filed an NDA, and for which we must complete our TRILOGY Phase 3 program and seek and receive regulatory approval prior to commercial launch. We do not anticipate filing our NDA until 2021 at the earliest. The results of our TRILOGY 1 trial did not meet its primary endpoint, and our ability to commercialize CaPre is now highly dependent on a positive, statistically significant outcome for our TRILOGY 2 trial, and a supportive position from the FDA to allow us to file an NDA by pooling data from both TRILOGY Phase 3 trials. We have invested significant effort and financial resources in researching and developing CaPre. Commercialization of CaPre will require substantial additional investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before we can generate any revenue from sales of CaPre, if it is ever approved by the FDA for commercialization.
We currently do not have any other prescription drug candidates in development, and so our business prospects depend entirely on the successful development, regulatory approval and commercialization of CaPre, which may never occur. Most prescription drug candidates never reach the clinical development stage and even those that do reach clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. If we are unable to successfully commercialize CaPre, we may never generate meaningful revenues. In addition, if CaPre reaches commercialization and there is low market demand for CaPre or the market for CaPre develops less rapidly than we anticipate, we may not have the ability to shift our resources to the development of alternative products.
| 39 |
We may not be able to obtain required regulatory approvals for CaPre.
We have limited experience in obtaining regulatory approvals, including approvals by the FDA and, as a company, we have no experience in obtaining regulatory approval of any product candidates. The research, testing, manufacturing, labeling, packaging, storage, sale, marketing, pricing, export, import and distribution of prescription drug products are subject to extensive regulation by the FDA in the United States and other regulatory authorities in other countries around the world, and regulations differ from country to country. We are not permitted to market CaPre in the United States until we receive approval of an NDA from the FDA, and similar restrictions apply in other countries. In the United States, the FDA generally requires the completion of preclinical testing and clinical trials for each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality and consistent manufacturing capabilities before an NDA is approved. Regulatory authorities in other jurisdictions impose similar requirements. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA, and even fewer are approved for commercialization. To date, we have not submitted an NDA for CaPre to the FDA or comparable applications to other regulatory authorities.
Our receipt of required regulatory approvals for CaPre is uncertain and subject to a number of risks, including:
| · | the FDA or comparable foreign regulatory authorities or independent institutional review boards may disagree with the design or implementation of our clinical trials; |
| · | we may not be able to provide acceptable evidence of the safety and efficacy of CaPre; |
| · | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA or other regulatory agencies for marketing approval; |
| · | the dosing of CaPre in a particular clinical trial may not be at an optimal level; |
| · | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to CaPre; |
| · | we may be unable to demonstrate that CaPre’s clinical and other benefits outweigh its safety risks; |
| · | the data collected from our clinical trials may not be sufficient to support the submission of an NDA for CaPre or to obtain regulatory approval for CaPre in the United States or elsewhere; |
| · | the FDA or comparable foreign regulatory authorities may not approve the manufacturing processes or facilities of third party manufacturers with which we contract for clinical and commercial supplies of CaPre; and |
| · | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
| 40 |
Furthermore, the preliminary topline data released in January 2020 relating to our TRILOGY 1 Phase 3 clinical trial was significantly impacted by an unusually large placebo effect. Our ongoing investigations into this unusually large placebo effect have not produced any definitive explanations, and there is no assurance that pooling of the data from our TRILOGY 1 and 2 trials will achieve statistical significance, or an outcome that is supported by the FDA. Furthermore, there is no assurance that any adjusted approach to analyzing data from our TRILOGY 1 and 2 trials would achieve statistical significance or allow for a filing of an NDA. For a further discussion of our TRILOGY 1 and 2 trials, see “Item 1. Business — Recent Developments.”
The FDA and other similar regulators have substantial discretion in the approval process and may refuse to accept our application or may decide that our data is insufficient for approval and require additional clinical trials, or preclinical or other studies for CaPre. If regulatory approval for CaPre is obtained in one jurisdiction that does not necessarily mean that CaPre will receive regulatory approval in all jurisdictions in which we seek approval. If we fail to obtain approval for CaPre in one or more jurisdictions, our ability to obtain approval in a different jurisdiction may be negatively affected.
Even if we receive regulatory approval for CaPre, it may just be for a limited indication.
If we obtain regulatory approval for CaPre, we will only be permitted to market it for the indication(s) approved by the FDA, and any such approval may put limits on the indicated uses or promotional claims we may make for it, or otherwise not permit labeling that sufficiently differentiates CaPre from competitive products with comparable therapeutic profiles. For example, while our initial objective is to seek regulatory approval for the treatment of sHTG, afterwards obtaining approval for CaPre to address mild to moderate HTG could greatly expand our potential market for CaPre. However, even if CaPre is approved for sHTG, it may never be approved for the treatment of mild to moderate HTG. In addition, any approval we receive for CaPre could contain significant use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If any regulatory approval for CaPre contains significant limits, we may not be able to obtain sufficient funding or generate meaningful revenue from CaPre or be able to continue developing, marketing or commercializing CaPre.
We may be unable to find successful strategic partnerships to develop and commercialize CaPre.
We intend to utilize an in-house team to market CaPre in the United States. We intend to seek co-development, licensing and/or marketing partnership opportunities with third parties for access to key markets around the world that we believe will complement or enhance our direct development and commercialization efforts for CaPre in the United States. Entering into potential partnerships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing shareholders or disrupt our management and business. Entering into partnerships could also delay the commercialization of CaPre, and our other future product candidates in those markets if we become dependent upon a strategic partner and that strategic partner does not prioritize the development of CaPre (or our future product candidates) relative to its other development activities. In addition, we face significant competition in seeking strategic partners, and the negotiation process is time-consuming and complex. We may not be successful in our efforts to establish a strategic partnership or other alternative commercial arrangements for CaPre on our anticipated timeline, or at all, because CaPre may be deemed to be at too early of a stage for collaborative effort, and/or third parties may not view CaPre as having the requisite potential to demonstrate safety, efficacy or product differentiation that will make it competitive. Even if we do enter into strategic partnerships, those partnerships may not achieve our objectives.
We may be unable to in-license and/or develop alternative product candidates.
To date, we have not commercialized any prescription drug candidates and, other than CaPre, we do not currently have any compounds in clinical trials, nonclinical testing, lead optimization or lead identification stages. If we fail to obtain regulatory approval for and successfully commercialize CaPre as a treatment for sHTG or any other indication, whether as a stand-alone therapy or in combination with other treatments, we would have to develop, acquire or license alternative product candidates or drug compounds to expand our product candidate pipeline beyond CaPre. In such a scenario, we may not be able to identify and develop or acquire product candidates that prove to be commercially successful, or to develop or acquire them on terms that are acceptable to us.
| 41 |
We may not be able to compete effectively against our competitors’ pharmaceutical products.
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to CaPre. It is probable that the number of companies seeking to develop products and therapies similar to CaPre will increase, particularly based on positive REDUCE-IT CVOT results by Amarin. In addition, on March 30, 2020, a federal district court ruled in favor of generic drug companies in patent litigation against two filers of abbreviated new drug applications for Amarin’s VASCEPA franchise in the United States. Amarin is now appealing that decision. A generic version of VASCEPA has now been approved by the FDA, but the timing of launch will be dependent on the outcome of Amarin’s appeal. More companies could be seeking to develop and produce products and therapies similar to CaPre. Many of our existing and potential competitors have substantially greater financial, technical and human resources than we do and may be better equipped to develop, manufacture and market products. These companies may develop and introduce products and processes competitive with or superior to CaPre. In addition, other technologies or products may be developed that have an entirely different approach or means of accomplishing the intended purposes of CaPre, which might render our technology and CaPre non-competitive or obsolete.
Our competitors in the United States and globally include large, well-established pharmaceutical companies, specialty pharmaceutical sales and marketing companies, and specialized cardiovascular treatment companies. GlaxoSmithKline plc, which sells LOVAZA, a prescription-only OM3 fatty acid indicated for patients with sHTG, was approved by the FDA in 2004 and has been available in the United States since 2005. Multiple generic versions of LOVAZA are now available in the United States. Amarin launched its prescription-only OM3 drug VASCEPA in 2013, and reached about a market share of approximately 20% by the end of 2015. Their U.S. market share in 2019 was estimated to have grown to more than 50%. In addition, EPANOVA (OM3-carboxylic acids), a free fatty acid form of OM3 (comprised of 55% EPA and 20% DHA), is FDA-approved for patients with sHTG. OMTRYG, another OM3 fatty acid composition developed by Trygg Pharma AS, received FDA approval for sHTG. Neither EPANOVA nor OMTRYG have yet been commercially launched. Matinas Biopharma recently started their development program for MAT9001, an OM-3 free fatty acid that consists primarily of EPA and docosapentaenoic acid. Other large companies with products that would compete indirectly with CaPre include AbbVie, Inc., which currently sells TRICOR and TRILIPIX for the treatment of sHTG, and NIASPAN, which is primarily used to raise HDL-C but is also used to lower TGs. Generic versions of TRICOR, TRILIPIX, and NIASPAN are also now available in the United States. In addition, we are aware of a number of other pharmaceutical companies that are developing non-OM3 products that, if approved and marketed, could compete with CaPre.
Even if it receives regulatory approval, CaPre will need to demonstrate compelling comparative advantages in efficacy, convenience, tolerability and safety to be commercially successful. Other competitive factors, including additional generic drug competition, could force us to lower prices or could result in reduced sales of CaPre. In addition, new products developed by others could emerge as competitors to CaPre. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
On March 30, 2020, the U.S. District Court for the District of Nevada ruled in favor of two generic companies (Hikma Pharmaceuticals plc and Dr. Reddy’s Laboratories Ltd) by deciding that Amarin’s patent claims for VASCEPA were invalid for being obvious in view of prior art. Amarin has filed an appeal, and both parties have requested the U.S. Court of Appeals for the Federal Circuit to review Amarin's appeal on an expedited schedule, with a decision expected later this year. Should Amarin lose this appeal, we would expect generic versions of VASCEPA to enter the market within the next year. This could have a negative impact on pricing much sooner than previously expected and could result in downward pressure on pricing for CaPre in order to get payer coverage.
CaPre could face competition from products for which no prescription is required.
If it receives regulatory approval, CaPre will be a prescription-only OM3. Mixtures of OM3 fatty acids are naturally occurring substances in various foods, including fatty fish. Lower potency and lower purity forms of OM3 fatty acids are also marketed by other non-pharmaceutical companies as dietary supplements or natural health products. Dietary supplements may generally be marketed without a lengthy FDA premarket review and approval process, and do not require a prescription. However, unlike drug products, manufacturers of dietary supplements are not permitted to make therapeutic claims for their products; dietary supplements may be marketed with claims describing how the product affects the structure or function of the body without premarket approval, but cannot expressly or implicitly represent that the dietary supplement will diagnose, cure, mitigate, treat, or prevent disease. We cannot be certain that physicians or consumers will view CaPre as superior to these alternatives or that physicians will be more likely to prescribe CaPre. If CaPre is not broadly covered by insurance, or the patient co-pay is significantly higher than the prices of commercially available OM3 fatty acids marketed by other companies as dietary supplements or natural health products, physicians may recommend these commercial alternatives instead of CaPre, or patients may elect on their own to take commercially available non-prescription OM3 fatty acids. Either of these outcomes could limit how we price CaPre and market adoption, and therefore negatively affect potential revenues.
| 42 |
Recent and future legal developments could make it more difficult and costly for us to obtain regulatory approvals for CaPre and negatively affect the prices we may charge.
In the United States and elsewhere, recent and proposed legal and regulatory changes to healthcare systems could prevent or delay our receipt of regulatory approval for CaPre, restrict or regulate our post-approval marketing activities, and adversely affect our ability to profitably sell CaPre. Proposals have also been made to expand post-approval requirements and to restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDA’s regulations, guidance or interpretations will be changed, or what impact any such changes will have, if any, on our ability to obtain regulatory approvals for CaPre. Further, the Centers for Medicare and Medicaid Services, or CMS, frequently changes product descriptors, coverage policies, product and service codes, payment methodologies and reimbursement values. Also, increased scrutiny by the U.S. Congress of the FDA’s approval process could significantly delay or prevent our receipt of regulatory approval for CaPre and subject us to more stringent product labeling and post-marketing testing and other requirements. Furthermore, for market approval in EU countries, a CVOT is currently required. These types of trials are large, costly, and follow patients for at least 5 years. There can be no guarantee that we will ever conduct an outcome trial to meet these requirements to market in the European Union.
In the United States, the Medicare Modernization Act, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The MMA expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, the MMA authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of the MMA and the expansion of federal coverage of drug products, we expect there will be additional pressure to contain and reduce healthcare costs. These healthcare cost reduction initiatives and other provisions of the MMA could decrease the coverage and price that we would receive for CaPre. While the MMA applies only to drug benefits for Medicare beneficiaries, private health insurance companies often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private health insurance companies.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the Health Care Reform Law), has broadened access to health insurance, reduced or constrained the growth of healthcare spending, enhanced remedies against fraud and abuse, added new transparency requirements for the healthcare and health insurance industries, imposed new taxes and fees on the health industry and imposed additional health policy reforms. Provisions of the Health Care Reform Law affecting pharmaceutical companies include requirements to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “donut hole”, and to pay an annual non-tax deductible fee to the federal government based on each company’s market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense. The Healthcare Reform Law also includes significant provisions that encourage state and federal law enforcement agencies to increase activities related to preventing, detecting and prosecuting those who commit fraud, waste and abuse in federal healthcare programs, including Medicare, Medicaid and Tricare.
Despite initiatives to invalidate the Health Care Reform Law, the U.S. Supreme Court has upheld key aspects of it. There is still uncertainty with respect to the impact the current U.S. presidential administration and the U.S. Congress may have, if any, and the effects of any changes will likely take time to unfold. As judicial challenges and legislative initiatives to modify, limit, or repeal the Healthcare Reform Law continue to evolve, the Health Care Reform Law may be significantly changed and we do not know whether any such changes could have significant negative financial impact on the development or potential profitability of CaPre. At this time, it remains unclear whether there will be any changes made to the Health Care Reform Law, whether to certain provisions or its entirety. The Health Care Reform Law or any replacement of it could continue to apply downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs. Additional federal healthcare reform measures could be adopted in the future limiting the amounts that federal and state governments will pay for healthcare products and services, which could negatively affect the value of CaPre and our ability to achieve profitability.
| 43 |
In Canada, most new patented drug prices are limited so that the cost of therapy is in the range of the cost of therapy for existing drugs sold in Canada used to treat the same disease. As a result:
| · | prices of drugs that show a moderate to substantial improvement, including breakthrough drugs are also restricted by a variety of tests; |
| · | existing patented drug prices cannot increase by more than the Canadian Consumer Price Index; and |
| · | the Canadian prices of patented medicines can never be the highest in the world. |
If CaPre receives regulatory approval in Canada, restrictions on the price we can charge there for CaPre could reduce the value of CaPre and our ability to generate revenue and achieve profitability.
In many jurisdictions outside the United States, a product candidate must be approved for health care reimbursement before it can be approved for sale. In some cases, the price that we intend to charge for CaPre will also be subject to approval. If we fail to comply with the regulatory requirements in our target international markets or to receive required marketing approvals, our potential market for CaPre will be reduced and our ability to realize the full market potential for CaPre will be harmed.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for CaPre, it is less likely that it will be widely used.
Even if CaPre is approved for sale by the appropriate regulatory authorities, market acceptance and sales of CaPre will depend on reimbursement policies and may be affected by future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will reimburse and establish payment levels. We cannot be certain that reimbursement will be available for CaPre. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize CaPre.
There may be significant delays in obtaining coverage and reimbursement for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or other regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution expenses. Interim reimbursement levels for new drugs, if applicable, may also be insufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of a drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower-cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for CaPre could have a material adverse effect on our operating results and our overall financial condition.
Even if we obtain FDA approval of CaPre, we may never obtain approval or commercialize it outside of the United States, which would limit our ability to realize CaPre’s full market potential.
In order to market CaPre outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval procedures vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approvals could result in significant delays, difficulties and costs for us and may require additional preclinical studies or clinical trials, which would be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of CaPre in those countries. In addition, our failure to obtain regulatory approval in any country may delay or have negative effects on the process for regulatory approval in other countries. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, our target market will be reduced and our ability to realize the full market potential of CaPre will be harmed.
| 44 |
If we or our third-party service providers fail to comply with healthcare laws and regulations or government price reporting laws, we could be subject to civil or criminal fines or penalties.
In addition to the FDA’s restrictions on marketing pharmaceutical products, several other types of federal and state healthcare fraud and abuse laws restrict marketing practices in the pharmaceutical industry. These laws include the U.S. Anti-Kickback Statute, U.S. False Claims Act and similar state laws. The U.S. Anti-Kickback Statute prohibits, among other things, offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, or ordering any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. A person or entity does not need to have actual knowledge of the U.S. Anti-Kickback Statute or special intent to violate the law in order to have committed a violation. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers and prescribers, dispensers, purchasers and formulary managers. The exemptions and safe harbors from prosecution are drawn narrowly and we may fail to meet all of the criteria for safe harbor protection from anti-kickback liability.
In addition, the Health Care Reform Law provides that the government may assert that a claim including items or services resulting from a violation of the U.S. Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the U.S. False Claims Act. Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. The “qui tam” provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government. These individuals, sometimes known as “relators” or, more commonly, as “whistleblowers”, may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a case brought under the federal False Claim Act. If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus attorneys’ fees and costs, and civil penalties of up to $21,563 for each separate false claim. Certain administrative sanctions, up to and including exclusion of an entity from participation in the federal healthcare programs, may also ensue.
Additional laws and regulations include:
| · | the federal Anti-Inducement Law (also known as the Civil Monetary Penalties Law), which prohibits a person from offering or transferring remuneration to a Medicare or State healthcare program beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of any item or service for which payment may be made, in whole or in part, by Medicare or a State healthcare program; |
| · | the Ethics in Patient Referrals Act of 1989, commonly referred to as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients for certain designated health services where that physician or family member has a financial relationship with the entity providing the designated health service, unless an exception applies; |
| · | the U.S. federal Health Insurance Portability and Accountability Act (HIPAA), as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), which created additional federal criminal statutes that prohibit, among other things, schemes to defraud healthcare programs and imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable health information, and requires notification to affected individuals and regulatory authorities of breaches of security of individually identifiable health information; |
| · | the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, to report annually to the CMS information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members, which is published in a searchable form on an annual basis; |
| 45 |
| · | federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies report or disclose pricing or other financial information and to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; and |
| · | the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on our business, results of operations and reputation. |
Over the past few years, a number of pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged prohibited promotional and marketing activities, such as providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. Most states also have statutes or regulations similar to the U.S. Anti-Kickback Statute and the U.S. False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment. Settlements of U.S. government litigation may include Corporate Integrity Agreements with commitments for monitoring, training, and reporting designed to prevent future violations.
Any action against us for an alleged or suspected violation of these laws could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with these laws and regulations may be costly to us in terms of money, time and resources. If we or any strategic partners, manufacturers or service providers fail to comply with these laws, we could be subject to enforcement actions, including:
| · | adverse regulatory inspection findings; |
| · | warning letters; |
| · | voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals; |
| · | restrictions on, or prohibitions against, marketing our products; |
| · | restrictions on, or prohibitions against, importation or exportation of our products; |
| · | suspension of review or refusal to approve pending applications or supplements to approved applications; |
| · | exclusion from participation in government-funded healthcare programs; |
| · | exclusion from eligibility for the award of government contracts for our products; |
| · | suspension or withdrawal of product approvals; |
| · | product seizures; |
| · | injunctions; and |
| · | civil and criminal penalties and fines. |
| 46 |
The research, development and manufacture of CaPre involves using potentially hazardous materials.
Our research and development activities relating to CaPre involve the controlled use of potentially hazardous substances, including chemical materials such as acetone. Our manufacturers for CaPre will be subject to federal, provincial, state and local laws and regulations in Canada, the United States and in other jurisdictions governing laboratory procedures and the use, manufacture, storage, handling and disposal of medical and hazardous materials. Although we believe that our procedures used by our contract manufacturing organizations for handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. If any such contamination or injury were to occur, we may incur liability or local, city, provincial, state or federal authorities may curtail the use of these materials and interrupt our business operations and the production of CaPre. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from medical or hazardous materials. Complying with environmental, health and safety laws and regulations is expensive, and current or future environmental regulations may impair our research, development and production efforts relating to CaPre, which could harm our business, prospects, financial condition or results of operations. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of potentially hazardous materials. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These laws and regulations may make it more difficult for us to conduct our research, development or production activities relating to CaPre and if we fail to comply with them, we could have substantial fines, penalties or other sanctions imposed against us.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to cease the sale, marketing and distribution of CaPre.
We face a potential risk of product liability associated with any future commercialization of CaPre or any other future product candidate we develop. For example, we may be sued if CaPre allegedly causes injury. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under U.S. state or Canadian provincial or other foreign consumer protection legislation. If we cannot successfully defend against product liability claims, we may incur substantial liabilities or may be required to cease the sale, marketing and distribution of CaPre. Even successful defense against product liability claims would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| · | decreased demand for CaPre or any future products that we may develop; |
| · | injury to our reputation; |
| · | costs to defend the related litigation; |
| · | a diversion of management’s time and our resources; |
| · | substantial monetary awards to consumers, trial participants or patients; |
| · | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| · | loss of revenue; |
| · | an inability to commercialize CaPre; and | |
| · | a decline in the price of our common shares. |
| 47 |
If we are unable to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims, the commercialization of CaPre or any other product candidates we develop could be hindered or prevented. We currently carry product liability insurance in the amount of $10.0 million in the aggregate. Any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. In the event of a successful product liability claim against us, we may have to pay from our own resources any amounts awarded by a court or negotiated in a settlement that exceed coverage limitations or that is not covered by our insurance, and we may not have, or be able to obtain, sufficient funds to pay such amounts.
We may not achieve our publicly announced milestones on time, or at all.
From time to time, we may publicly announce the timing of certain events that we expect to occur, such as the anticipated timing of results from our clinical trials and the timing of an upcoming NDA filing. These statements are forward-looking and are based on the best estimate of management at the time relating to the occurrence of the events. However, the actual timing of these events may differ from what has been publicly disclosed. The timing of events such as completion of a clinical trial, discovery of a new product candidate, filing of an application to obtain regulatory approval, beginning of commercialization of products, completion of a strategic partnership, or announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. For example, we cannot provide assurances that our current estimate of the completion date for our TRILOGY Phase 3 program will be accurate, that we will not require additional studies to submit an NDA, that we will make regulatory submissions or receive regulatory approvals as planned, that we will be able to adhere to plans for the scale-up of manufacturing and launch of CaPre, or that our TRILOGY Phase 3 clinical trials for CaPre will achieve all or any of their primary and secondary endpoints. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier or a distribution partner or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously-announced milestones could have a material adverse effect on our business, financial condition or operating results and the trading price of our common shares.
We may be subject to foreign exchange rate fluctuations.
Our reporting currency is the U.S. dollar. However, many of our expenses, such as CaPre’s chief manufacturing organization’s production activities and certain CRO arrangements for our TRILOGY Phase 3 program, currently are and/or are expected to be, denominated in foreign currencies, including Canadian dollars and European euros. As we previously completed financings in both Canadian and U.S. dollars, both currencies are maintained and used to make required payments in the applicable currency. Though we plan to implement measures designed to reduce our foreign exchange rate exposure, the U.S. dollar/Canadian dollar and U.S. dollar /European euro exchange rates have fluctuated significantly in the recent past and may continue to do so, which could have a material adverse effect on our business, financial position and results of operations.
In the past, Neptune supplied us with the RKO needed to produce CaPre for all of our clinical and non-clinical trials, including the RKO that was needed to supply our TRILOGY Phase 3 program. In 2019 we validated a new RKO supplier and we are now evaluating additional suppliers for on going commercial supply.
RKO is the starting material used by Acasti to make CaPre, which is then further processed via a series of complex and proprietary extraction and purification manufacturing steps to produce the active pharmaceutical ingredient, or API, for CaPre. We sourced all of our RKO from Neptune in the past to produce CaPre for our clinical programs. However, in light of Neptune’s sale of its krill oil business and inventory to Aker in August 2017, we immediately began validating several alternative suppliers of RKO. In November 2019, we announced that we had signed a two-year, fixed price supply agreement with Aker to provide RKO for the purpose of building commercial lots of CaPre. This agreement is intended to ensure an adequate RKO supply to meet our anticipated raw material needs through at least mid-2021, including for the scale-up of production of API to build CaPre inventory for a potential commercial launch.
| 48 |
While we believe that there are alternative suppliers of RKO that could be readily available and meet our specifications, we do not have enough experience with any one of them to guarantee that these alternative suppliers will be of comparable quality to the RKO previously provided by Neptune and now, Aker, which could negatively affect the cost of CaPre. Our reliance on third-party suppliers for RKO exposes us to risks such as potential fluctuations in supply and reduced control over our production costs and delivery schedules for CaPre.
CaPre may cause or be perceived to cause undesirable side effects or have other properties that could delay or prevent its regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Many of the patients that we enrolled in our TRILOGY Phase 3 clinical trials may have pre-existing disorders. While such disorders may lead to serious adverse events during the clinical trial that may be found to be unrelated to CaPre, such events may create a negative safety perception and adversely impact market acceptance of CaPre following any approval. The safety profile of CaPre in our TRILOGY 1 trial was similar to placebo, as there was no significant difference in treatment-related serious adverse events in the trial. Safety results for our TRILOGY 2 trial remain blinded.
While patient participation in our TRILOGY Phase 3 program has been completed, it is still possible that a future study conducted by a collaborator or third party researcher may identify undesirable side effects. If, following any approval of CaPre or another product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified during the clinical trial phase, any of the following adverse events could occur:
| · | regulatory authorities may withdraw their approval of the product or seize the product; |
| · | we, or any future collaborators or third party researcher, may need to recall the product, or be required to change the way the product is administered or conduct additional clinical trials; |
| · | restrictions may be imposed on the marketing of, or the manufacturing processes for the product; |
| · | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| · | regulatory authorities may require the addition of labeling statements; |
| · | we, or any future collaborators, may be required to issue a communication outlining the risks of the previously unidentified side effects for distribution to patients; |
| · | we, or any future collaborators, could be sued and held liable for harm caused to patients; |
| · | the product may become less competitive; and |
| · | our reputation may suffer. |
Any of these events could harm our business and operations and could negatively impact our share price.
Risks Related to Intellectual Property
In addition to our own patents, CaPre is covered by patents that are sublicensed to us by Neptune and Aker.
In addition to our proprietary issued patents and pending patent applications, pursuant to a license agreement we entered into with Neptune in August 2008, which was later amended on February 9, 2009 and March 7, 2013 (the “License Agreement”), we have an exclusive license to use certain intellectual property developed by Neptune and now owned by Aker, to develop, manufacture and commercialize CaPre, and our novel and APIs for use in pharmaceutical and medical food applications in the cardiovascular field. Aker has granted to Neptune the right to sublicense to us certain intellectual property as necessary to allow us to maintain its license grant under the License Agreement. Accordingly, the exclusive license granted to us under the License Agreement remains in full force.
| 49 |
Disputes may arise between us and Aker regarding the intellectual property that is subject to the License Agreement, including with respect to the scope of rights granted under the License Agreement and other interpretation-related issues and our right to sublicense patent and other rights to third parties under collaborative development relationships.
It is difficult and costly to protect our intellectual property rights.
The success of our business will largely depend on our ability to:
| · | obtain and maintain our patents and trade secret protections and operate without infringing the intellectual proprietary rights of third parties; |
| · | successfully defend our patents, including enforcing our licensed patents against third-party challenges; and |
| · | successfully enforce our patents against third party competitors. |
It is possible that our patents and/or proprietary technologies in the future could be circumvented through the adoption of competitive, though non-infringing, processes or products. The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in interpretations of patent laws may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowable or enforceable in our patents, or of patents licensed to us.
We face risks that:
| · | our rights under our U.S., Canadian or foreign patents or other licensed patents that other third parties license to us could be curtailed; |
| · | we may not be the first inventor of inventions covered by our issued patents or pending applications or be the first to file patent applications for those inventions; |
| · | our pending or future patent applications may not be issued with the breadth of claim coverage sought by us, or be issued at all; |
| · | our competitors could independently develop or patent technologies that are substantially equivalent or superior to our technologies; |
| · | our trade secrets could be learned independently by our competitors; |
| · | the steps we take to protect our intellectual property may not be adequate; and |
| · | effective patent, trademark, copyright and trade secret protection may be unavailable, limited or not sought by us in some foreign countries. |
Further, patents have a limited lifespan. In the United States, a patent generally expires 20 years after it is filed (or 20 years after the filing date of the first non-provisional U.S. patent application to which it claims priority). While extensions may be available, the life of a patent, and the protection it affords, is limited. Without patent protection for CaPre or any other of our future product candidates, we may be open to competition from generic versions of CaPre or our other future product candidates. Further, the extensive period of time between patent filing and regulatory approval for a product candidate limits the time during which we can market that product candidate under patent protection. Patents owned by third parties could have priority over patent applications filed or in-licensed by us, or we or our licensors could become involved in interference, opposition or invalidity proceedings before U.S., Canadian or foreign patent offices. The cost of defending and enforcing our patent rights against infringement charges by other patent holders may be significant and could limit our operations.
| 50 |
CaPre may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts.
Our success depends in part on avoiding infringement of the proprietary technologies of others. The pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Identification of third party patent rights that may be relevant to our proprietary or licensed technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Additionally, because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by our development and commercialization of CaPre or any other future product candidate. There may be certain issued patents and patent applications claiming subject matter that we may be required to license in order to research, develop or commercialize CaPre, and any such patents and patent applications may not be available to license on commercially reasonable terms, or at all. If claims of patent infringement are asserted by third parties against us, they could be time-consuming and may:
| · | result in costly litigation; |
| · | divert the time and attention of our technical personnel and management; |
| · | delay future clinical trials for CaPre; |
| · | prevent us from commercializing CaPre until the asserted patent expires or is held finally invalid or not infringed in court; |
| · | require us to cease or to modify our use of the technology and/or develop non-infringing technology; or |
| · | require us to enter into royalty or licensing agreements. |
Others may hold proprietary rights that could prevent CaPre from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to CaPre or our processes could subject us to potential liability for damages and require us to obtain a license to continue to manufacture or market CaPre or any other future prescription drug candidates. We might not prevail in any such actions or if any license is required under any of these patents it may not be available on commercially acceptable terms, if at all.
Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. We could be forced to redesign CaPre or any other future product candidates or processes to avoid infringement.
In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
A number of companies, including several major pharmaceutical companies, have conducted research on pharmaceutical uses of OM3 fatty acids, which has resulted in the filing of many patent applications related to this research. We are aware of third-party U.S., Canadian and other foreign patents that contain broad claims related to methods of using these general types of compounds, which may be construed to include potential uses of CaPre. If we were to challenge the validity of these or any other issued U.S., Canadian or other foreign patents in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. and Canadian or other foreign patent. This means that, in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the other party’s patent’s claims. If we were to challenge the validity of any issued U.S. patent, for example, in an administrative trial before the Patent Trial and Appeal Board in the United States Patent and Trademark Office, or USPTO, we would have to prove that the claims are unpatentable by a preponderance of the evidence. If there are disputes over our intellectual property rights, a jury and/or court may not find in our favor on questions of infringement, validity or enforceability.
| 51 |
If we do not protect our trademark for CaPre or any new trademark that is developed for CaPre, we may not be able to build name recognition in our markets of interest.
We have registered CaPre as a trademark in several jurisdictions. Our trademark, or any new mark that is developed for CaPre may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to this trademark or may be forced to stop using this name, which we need for name recognition by potential strategic partners and customers. If we are unable to establish name recognition based on our trademark, we may not be able to compete effectively, and our business may be adversely affected.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. If we or our licensors were to initiate legal proceedings against a third party to enforce a patent covering CaPre or our technology, the defendant could counterclaim that our or our licensor’s patent is invalid or unenforceable. In patent litigation, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements; for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the patent office, such as the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we or our licensors and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on CaPre or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
In addition, in an infringement proceeding, a court may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
Interference proceedings provoked by third parties or brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all. Litigation or interference proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States and Canada. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
| 52 |
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect CaPre and any of our other future product candidates.
Numerous recent changes to the patent laws and proposed changes to the rules of the various patent offices around the world may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. These changes may lead to increasing uncertainty with regard to the scope and value of our issued patents and to our ability to obtain patents in the future.
Once granted, patents may remain open to opposition, re-examination, post-grant review, inter partes review, nullification derivation and opposition proceedings in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against the initial grant. In the course of any such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims attacked, or may lose the allowed or granted claims altogether. Depending on decisions by authorities in various jurisdictions, the laws and regulations governing patents could change in unpredictable ways that may weaken our and our licensors’ ability to obtain new patents or to enforce existing patents we and our licensors or partners may obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Relating to Our Common Shares
The price of our common shares may be volatile.
Market prices for pharmaceutical companies can fluctuate significantly. Factors such as the announcement to the public or in various scientific or industry forums of technological innovations; new commercial products; patents or exclusive rights obtained by us or others; disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; the commencement, enrollment or announcement of results of clinical trials we conduct, or changes in the development status of our product candidates; results or delays of pre-clinical and clinical studies by us or others; any delay in our regulatory filings for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings; a change of regulations; additions or departures of key scientific or management personnel; overall performance of the equity markets; general political and economic conditions; publications; failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public; research reports or positive or negative recommendations or withdrawal of research coverage by securities analysts; actual or anticipated variations in quarterly operating results; announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; public concerns over the risks of pharmaceutical products and dietary supplements; unanticipated serious safety concerns related to the use of CaPre; the ability to finance, future sales of securities by us or our shareholders; and many other factors, many of which are beyond our control, could have considerable effects on the price of our common shares. The price of our common shares has fluctuated significantly in the past and there can be no assurance that the market price of our common shares will not experience significant fluctuations in the future.
| 53 |
In addition, pharmaceutical companies often experience extreme price and volume fluctuations that are unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may negatively affect the market price of our common shares, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against pharmaceutical companies following periods of volatility in the market price of their securities. This type of litigation, if instituted against us, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We will need to raise additional capital in order to execute on our business plan. We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. The incurrence of indebtedness by us would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us.
The market price of our common shares could decline as a result of operating results falling below the expectations of investors or fluctuations in operating results each quarter.
Our net losses and expenses may fluctuate significantly and any failure to meet financial or clinical expectations may disappoint securities analysts or investors and result in a decline in the price of our common shares. Our net losses and expenses have fluctuated in the past and are likely to do so in the future. The market price of our common shares has fluctuated significantly in the past and may continue to do so. Some of the factors that could cause the market price for our common shares to fluctuate include the following:
| · | results of preclinical studies and clinical trials, or the addition or termination of preclinical studies, clinical trials or funding support; |
| · | the fluctuations in valuation of our derivative warrant liabilities; |
| · | the timing of the release of results from any preclinical studies and clinical trials; |
| · | an inability to complete product development in a timely manner that results in a failure or delay in receiving the required regulatory responses, approvals or allowances to commercialize product candidates; |
| · | the timing of regulatory responses, submissions and approvals; |
| · | the timing and willingness of any current or future collaborators to invest the resources necessary to commercialize our products; |
| · | the outcome of any litigation; |
| · | changes in foreign currency fluctuations; |
| · | competition; |
| · | the timing of achievement and the receipt of milestone payments from current or future third parties; |
| 54 |
| · | failure to enter into new or the expiration or termination of current agreements with third parties; |
| · | failure to introduce our products to the market in a manner that generates anticipated revenues; |
| · | execution of any new collaboration, licensing or similar arrangement, and the timing of payments we may make or receive under such existing or future arrangements or the termination or modification of any such existing or future arrangements; |
| · | any intellectual property infringement lawsuit or opposition against us or our competition that could have a negative impact on the OM3 space, interference or cancellation proceeding in which we may become involved; |
| · | additions and departures of key personnel; |
| · | strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; |
| · | if any of our product candidates receives regulatory, or fails to receive approval, market acceptance and demand for such product candidates; |
| · | regulatory developments affecting our product candidates or those of our competitors; and |
| · | changes in general market and economic conditions. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the market price of our common shares could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the market price of our common shares to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
There can be no assurance that an active market for our common shares will be sustained.
There can be no assurance that an active market for our common shares will be sustained. Holders of common shares may be unable to sell their investments on satisfactory terms. As a result of any risk factor discussed herein, the market price of our common shares at any given point in time may not accurately reflect our long-term value. Furthermore, responding to these risk factors could result in substantial costs and divert management’s attention and resources. Substantial and potentially permanent declines in the value of our common shares may adversely affect the liquidity of the market for our common shares.
Other factors unrelated to our performance that may have an effect on the price and liquidity of our common shares include: positive or negative industry or competitor news; extent of analyst coverage; lessening in trading volume and general market interest in our common shares; the size of our public float; and any event resulting in a delisting of our common shares.
A large number of common shares may be issued and subsequently sold upon the exercise of existing warrants. The sale or availability for sale of existing warrants or other securities convertible into common shares may depress the price of our common shares.
As of March 31, 2020, there were 15.9 million common shares issuable under outstanding warrants at various exercise prices. To the extent that holders of existing warrants sell common shares issued upon the exercise of warrants, the market price of our common shares may decrease due to the additional selling pressure in the market. The risk of dilution from issuances of common shares underlying existing warrants may cause shareholders to sell their common shares, which could further contribute to any decline in our common share market price.
Any downward pressure on the price of our common shares caused by the sale of common shares issued upon the exercise of existing warrants could encourage short sales by third parties. In a short sale, a prospective seller borrows common shares from a shareholder or broker and sells the borrowed common shares. The prospective seller anticipates that the common share price will decline, at which time the seller can purchase common shares at a lower price for delivery back to the lender. The seller profits when the common share price declines because it is purchasing common shares at a price lower than the sale price of the borrowed common shares. Such short sales of common shares could place downward pressure on the price of our common shares by increasing the number of common shares being sold, which could lead to a decline in the market price of our common shares.
| 55 |
We do not currently intend to pay any cash dividends on our common shares in the foreseeable future.
We have never paid any cash dividends on our common shares and we do not anticipate paying any cash dividends on our common shares in the foreseeable future because, among other reasons, we currently intend to retain any future earnings to finance our business. The future payment of cash dividends will be dependent on factors such as cash on hand and achieving profitability, the financial requirements to fund growth, our general financial condition and other factors our board of directors may consider appropriate in the circumstances. Until we pay cash dividends, which we may never do, our shareholders will not be able to receive a return on their common shares unless they sell them. See “Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities — Dividends.”
If we fail to meet applicable listing requirements, the NASDAQ Stock Market or the TSXV may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline.
Our common shares are currently listed on the NASDAQ Stock Market and the TSXV, but we cannot assure you that our securities will continue to be listed on the NASDAQ Stock Market and the TSXV in the future. In the past, we have received notices from the NASDAQ Stock Market that we have not been in compliance with its continued listing standards, and we have taken responsive actions and regained compliance.
On February 28, 2020, we received written notification from the NASDAQ Listing Qualifications Department for failing to maintain a minimum bid price of $1.00 per share for the preceding 30 consecutive business days, as required by NASDAQ Listing Rule 5550(a)(2) – bid price (the “Minimum Bid Price Rule”). The NASDAQ notification has no immediate effect on the listing of our common shares. Under NASDAQ Listing Rule 5810(c)(3)(A) – compliance period, we have 180 calendar days to regain compliance.
On April 17, 2020, we were informed that NASDAQ had granted temporary regulatory relief related to its minimum bid price requirement due to the COVID-19 pandemic for all NASDAQ-listed companies. As a result of the announced regulatory relief, we now have until at least November 9, 2020 to regain compliance. We have not regained compliance to date.
If at any time over this relief period the bid price of our common shares closes at $1.00 per share or more for a minimum of ten (10) consecutive business days, NASDAQ will provide written confirmation of compliance and the matter will be closed. If we do not regain compliance within the relief period, but otherwise meet the continued listing requirements for market value of publicly-held shares and all other initial listing standards for the NASDAQ Listing Rule 5505 – Capital Market criteria, except for the Minimum Bid Price Rule, we may be eligible for an additional 180 calendar days to regain compliance. If we are not granted additional time, then our common shares will be subject to delisting, at which time we may appeal the delisting determination to a NASDAQ Hearings Panel.
If we fail to comply with listing standards and the NASDAQ Stock Market or TSXV delists our common shares, we and our shareholders could face significant material adverse consequences, including:
| · | a limited availability of market quotations for our common shares; |
| · | reduced liquidity for our common shares; |
| · | a determination that our common shares are “penny stock”, which would require brokers trading in our common shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our common shares; |
| 56 |
| · | a limited amount of news about us and analyst coverage of us; and |
| · | a decreased ability for us to issue additional equity securities or obtain additional equity or debt financing in the future. |
We may pursue opportunities or transactions that adversely affect our business and financial condition.
Our management, in the ordinary course of our business, regularly explores potential strategic opportunities and transactions. These opportunities and transactions may include strategic joint venture relationships, significant debt or equity investments in us by third parties, the acquisition or disposition of material assets, the licensing, acquisition or disposition of material intellectual property, the development of new drug candidates or new applications for CaPre, significant distribution arrangements, the sale of our common shares and other similar opportunities and transactions. The public announcement of any of these or similar strategic opportunities or transactions might have a significant effect on the price of our common shares. Our policy is to not publicly disclose the pursuit of a potential strategic opportunity or transaction unless we are required to do so by applicable law, including applicable securities laws relating to periodic disclosure obligations. There can be no assurance that investors who buy or sell common shares are doing so at a time when we are not pursuing a particular strategic opportunity or transaction that, when announced, would have a significant effect on the price of our common shares.
In addition, any such future corporate development may be accompanied by certain risks, including exposure to unknown liabilities of the strategic opportunities and transactions, higher than anticipated transaction costs and expenses, the difficulty and expense of integrating operations and personnel of any acquired companies, disruption of our ongoing business, diversion of management’s time and attention, and possible dilution to shareholders. We may not be able to successfully overcome these risks and other problems associated with any future acquisitions and this may adversely affect our business and financial condition.
We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies.
We are a “smaller reporting company” under the SEC’s disclosure rules, meaning that we have either:
| · | a public float of less than $250 million; or |
| · | annual revenues of less than $100 million during the most recently completed fiscal year; and |
| o | no public float; or |
| o | a public float of less than $700 million. |
As a smaller reporting company, we are permitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a smaller reporting company, the scaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies.
If investors consider our common shares less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common shares and our share price may be more volatile.
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We are a non-accelerated filer under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and we are not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our common shares less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and trading price for our common shares may be negatively affected.
| 57 |
U.S. investors may be unable to enforce certain judgments.
We are a company existing under the Business Corporations Act (Québec). Some of our directors and officers are residents of Canada, and substantially all of our assets are currently located outside the United States. As a result, it may be difficult to effect service within the United States upon us or upon some of our directors and officers. Execution by U.S. courts of any judgment obtained against us or any of our directors or officers in U.S. courts may be limited to assets located in the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of U.S. courts predicated upon civil liability of us and our directors and executive officers under the U.S. federal securities laws. There may be doubt as to the enforceability in Canada against non-U.S. entities or their controlling persons, directors and officers who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities predicated solely upon U.S. federal or state securities laws.
There is a significant risk that we may be classified as a PFIC for U.S. federal income tax purposes.
Current or potential investors in our common shares who are U.S. Holders (as defined below) should be aware that, based on our most recent financial statements and projections and given uncertainty regarding the composition of our future income and assets, there is a significant risk that we may have been classified as a “passive foreign investment company” or “PFIC” for the 2020 taxable year and may be classified as a PFIC for our current taxable year and possibly subsequent years. If we are a PFIC for any year during a U.S. Holder’s holding period of our common shares, then such U.S. taxpayer generally will be required to treat any gain realized upon a disposition of such common shares or any so-called “excess distribution” received on such common shares, as ordinary income (with a portion subject to tax at the highest rate in effect), and to pay an interest charge on a portion of such gain or excess distribution. In certain circumstances, the sum of the tax and the interest charge may exceed the total amount of proceeds realized on the disposition, or the amount of excess distribution received, by the U.S. Holder. Subject to certain limitations, a timely and effective QEF Election (as defined below) under Section 1295 of the U.S. Internal Revenue Code of 1986, as amended, or the Code, or a Mark-to-Market Election (as defined below) under Section 1296 of the Code may be made with respect to the common shares. A U.S. Holder who makes a timely and effective QEF Election generally must report on a current basis its share of our net capital gain and ordinary earnings for any year in which we are a PFIC, whether or not we distribute any amounts to our shareholders. A U.S. Holder who makes the Mark-to-Market Election generally must include as ordinary income each year the excess of the fair market value of their common shares over the holder’s basis therein. This paragraph is qualified in its entirety by the discussion under the heading “Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities - U.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common Shares - Passive Foreign Investment Company Rules.” Each current or potential investor who is a U.S. Holder should consult its own tax advisor regarding the U.S. federal, state and local, and non-U.S. tax consequences of the acquisition, ownership, and disposition of our common shares, the U.S. federal tax consequences of the PFIC rules, and the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
Our change from foreign private issuer to U.S. domestic issuer status may result in additional costs to us.
September 30, 2019, we no longer qualified as a “foreign private issuer” as defined in Rule 405 under the U.S. Securities Act of 1933, as amended, and Rule 3b-4 of the Exchange Act. As a foreign private issuer, we were exempt from certain provisions under U.S. federal securities laws applicable to U.S. public companies. We are now considered a U.S. domestic issuer and are subject to increased compliance obligations under the Exchange Act. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly more than the costs we incurred as a foreign private issuer.
As a U.S. domestic filer, we are no longer exempt from the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q and current reports on Form 8-K and filings of proxy statements with the SEC; the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations, in respect of shares registered under the Exchange Act; the provisions of Regulation FD aimed at preventing issuers from making selective disclosures of material information; and the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and establishing insider liability for profits realized from any “short-swing” trading transaction (a purchase and sale, or sale and purchase, of the issuer’s equity securities within less than six months).
| 58 |
We are also no longer eligible to rely upon exemptions from certain corporate governance requirements that are available to foreign private issuers or to benefit from other accommodations for foreign private issuers under the rules of the SEC and NASDAQ, which may involve additional costs.
| Item 1B. | Unresolved Staff Comments |
Not applicable.
| Item 2. | Properties |
Our head office and operations are located at 545, Promenade Centropolis, Suite 100, Laval, Québec, Canada, H7T 0A3 and our research and development and quality control laboratory is located at Espace Lab, 2650 Maximilien-Chagnon, Sherbrooke, Québec, Canada, J1E 0M8. We currently lease our office and laboratory space. We do not own our own manufacturing facility for the production of CaPre; however, we do own the proprietary equipment for producing the API and drug product. We currently do not have plans to develop our own manufacturing facility. However, this could change in the foreseeable future, as we consider the most cost-effective approaches to producing CaPre while ensuring the highest level of quality. We currently depend on third party suppliers and manufacturers to produce our required RKO and drug substance and products. If CaPre is approved for distribution by the FDA, we initially expect to rely on cGMP-compliant third parties to manufacture NKPL66, which is the API in CaPre, and to encapsulate, bottle and package clinical supplies of CaPre.
| Item 3. | Legal Proceedings |
Due to the fact that a portion of our intellectual property rights are licensed to us by Neptune/Aker, we rely on Neptune/Aker to protect a certain of the intellectual property rights that we use under our license agreement with Neptune/Aker. Neptune/Aker are engaged in a number of legal actions related to their intellectual property.
On May 10, 2019, we announced the settlement regarding legal claims made by our former chief executive officer with respect to the termination of his employment. Pursuant to the settlement agreement, we agreed to issue 900,000 common shares to the former CEO and also agreed to reimburse the former CEO for nominal legal fees.
Pursuant to the settlement agreement, we received a full and final release from the former CEO on all proceedings in connection with the termination of his employment.
| Item 4. | Mine Safety Disclosures |
Not applicable.
| 59 |
| Item 5. | Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common shares are traded on The Nasdaq Capital Market and the TSX Venture Exchange under the symbol “ACST.”
Holders
As of June 24, 2020, there were approximately 85 holders of record of our common shares. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We do not anticipate paying any cash dividend on the common shares in the foreseeable future. We presently intend to retain future earnings to finance the expansion and growth of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors the board of directors deems relevant. In addition, the terms of any future debt or credit facility may preclude us from paying dividends.
Taxation
The following is a summary of certain U.S. federal income tax considerations arising from and relating to the acquisition, ownership, and disposition of our common shares to a U.S. Holder (as defined below) as capital assets.
This summary provides only general information and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder as a result of the acquisition, ownership, and disposition of our common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences applicable to that U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Each U.S. Holder should consult its own tax advisor regarding the U.S. federal, state and local, and non-U.S. tax consequences arising from or relating to the acquisition, ownership, and disposition of our common shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service, or IRS, has been requested, or will be obtained, regarding the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Disclosure
Authorities
This summary is based on the Code, U.S. Treasury Regulations promulgated thereunder (whether final, temporary or proposed), published IRS rulings, judicial decisions, published administrative positions of the IRS, and the Convention between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the Canada-U.S. Tax Treaty), in each case, as in effect as of the date of this report. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive basis. Unless otherwise discussed, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
| 60 |
U.S. Holders
For purposes of this summary, a “U.S. Holder” is a beneficial owner of common shares that, for U.S. federal income tax purposes, is (a) an individual who is a citizen or resident of the United States, (b) a corporation, or other entity classified as a corporation for U.S. federal income tax purposes, that is created or organized in or under the laws of the U.S., any state in the United States or the District of Columbia, (c) an estate if the income of such estate is subject to U.S. federal income tax regardless of the source of such income, or (d) a trust if (i) such trust has validly elected to be treated as a U.S. person for U.S. federal income tax purposes or (ii) a U.S. court is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of such trust.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax consequences applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following U.S. Holders: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax deferred accounts; (b) U.S. Holders that are financial institutions, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are dealers in securities or currencies or U.S. Holders that are traders in securities that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders subject to the alternative minimum tax provisions of the Code; (f) U.S. Holders that own common shares as part of a straddle, hedging transaction, conversion transaction, integrated transaction, constructive sale, or other arrangement involving more than one position; (g) U.S. Holders that acquired common shares through the exercise of employee stock options or otherwise as compensation for services; (h) U.S. Holders that hold common shares other than as a capital asset within the meaning of Section 1221 of the Code; (i) U.S. Holders that beneficially own (directly, indirectly or by attribution) 10% or more of our equity securities (by vote or value); and (j) U.S. expatriates. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of the common shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds common shares, the U.S. federal income tax consequences to that partnership and the partners of that partnership generally will depend on the activities of the partnership and the status of the partners. Partners of entities that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership and disposition of the common shares.
Tax Consequences Other than U.S. Federal Income Tax Consequences Not Addressed
This summary does not address the U.S. estate and gift, alternative minimum, state, local or non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares. Each U.S. Holder should consult its own tax advisor regarding the U.S. estate and gift, alternative minimum, state, local and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of our common shares.
U.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common Shares
Distributions on Common Shares
Subject to the discussion under “—Passive Foreign Investment Company Rules” below, a U.S. Holder that receives a distribution, including a constructive distribution or a taxable stock distribution, with respect to the common shares generally will be required to include the amount of that distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of our current or accumulated “earnings and profits” (as computed for U.S. federal income tax purposes). To the extent that a distribution exceeds our current and accumulated “earnings and profits”, the excess amount will be treated (a) first, as a tax-free return of capital to the extent of a U.S. Holder’s adjusted tax basis in the common shares with respect to which the distribution is made (resulting in a corresponding reduction in the tax basis of those common shares) and, (b) thereafter, as gain from the sale or exchange of those common shares (see the more detailed discussion at “—Disposition of Common Shares” below). We do not intend to calculate our current or accumulated earnings and profits for U.S. federal income tax purposes and, therefore, will not be able to provide U.S. Holders with that information. U.S. Holders should therefore assume that any distribution by us with respect to our common shares will constitute a dividend. However, U.S. Holders should consult their own tax advisors regarding whether distributions from us should be treated as dividends for U.S. federal income tax purposes. Dividends paid on our common shares generally will not be eligible for the “dividends received deduction” allowed to corporations under the Code with respect to dividends received from U.S. corporations.
| 61 |
A dividend paid by us generally will be taxed at the preferential tax rates applicable to long-term capital gains if, among other requirements, (a) we are a “qualified foreign corporation” (as defined below), (b) the U.S. Holder receiving the dividend is an individual, estate, or trust, and (c) the dividend is paid on common shares that have been held by the U.S. Holder for at least 61 days during the 121-day period beginning 60 days before the “ex-dividend date” (i.e., the first date that a purchaser of the common shares will not be entitled to receive the dividend).
For purposes of the rules described in the preceding paragraph, we generally will be a “qualified foreign corporation”, or a QFC, if (a) we are eligible for the benefits of the Canada-U.S. Tax Treaty, or (b) our common shares are readily tradable on an established securities market in the United States, within the meaning provided in the Code. However, even if we satisfy one or more of the requirements, we will not be treated as a QFC if we are classified as a PFIC (as discussed below) for the taxable year during which we pay the applicable dividend or for the preceding taxable year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of those rules to them in their particular circumstances. Even if we satisfy one or more of the requirements, as noted below, there can be no assurance that we will not be a PFIC in the current taxable year, or become a PFIC in the future. Thus, there can be no assurance that we will qualify as a QFC.
Disposition of Common Shares
Subject to the discussion under “—Passive Foreign Investment Company Rules” below, a U.S. Holder will recognize gain or loss on the sale or other taxable disposition of common shares (that is treated as a sale or exchange for U.S. federal income tax purposes) equal to the difference, if any, between (a) the U.S. dollar value of the amount realized on the date of the sale or disposition and (b) the U.S. Holder’s adjusted tax basis (determined in U.S. dollars) in the common shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if the common shares are held for more than one year. A U.S. Holder's initial tax basis in the common shares generally will equal the U.S. dollar cost of such common shares. Each U.S. Holder should consult its own tax advisor as to the tax treatment of dispositions of common shares in exchange for Canadian dollars.
Preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to complex limitations.
Passive Foreign Investment Company Rules
If we are or become a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares.
Passive Foreign Investment Company Status.
Special, generally unfavorable, rules apply to the ownership and disposition of the stock of a PFIC. For U.S. federal income tax purposes, a non-U.S. corporation is classified as a PFIC if:
| · | at least 75% of its gross income for the taxable year is “passive” income (referred to as the “income test”); or |
| · | at least 50% of the average value of its assets held during the taxable year is attributable to assets that produce passive income or are held for the production of passive income (referred to as the “asset test”). |
| 62 |
Passive income generally includes the following types of income:
| · | dividends, royalties, rents, annuities, interest, and income equivalent to interest; and |
| · | net gains from the sale or exchange of property that gives rise to dividends, interest, royalties, rents, or annuities and certain gains from the commodities transactions. |
In determining whether we are a PFIC, we will be required to take into account a pro rata portion of the income and assets of each corporation in which we own, directly or indirectly, at least 25% by value.
As described above, PFIC status of a non-U.S. corporation depends on the relative values of certain categories of assets and the relative amount of certain kinds of income for a taxable year. Therefore, our status as a PFIC for any given taxable year depends upon the financial results for such year and upon relative valuations, which are subject to change and beyond our ability to predict or control. Based on our most recent financial statements and projections and given uncertainty regarding the composition of our future income and assets, there is a significant risk that we may have been classified as a PFIC for the 2020 taxable year and may be classified as a PFIC for our current taxable year and possibly subsequent years. However, PFIC status is fundamentally factual in nature, depends on the application of complex U.S. federal income tax rules (which are subject to differing interpretations), generally cannot be determined until the close of the taxable year in question and is determined annually. Accordingly, there can be no assurance that we will not be a PFIC in our current taxable year or subsequent years. The PFIC rules are complex, and each U.S. Holder should consult its tax advisor regarding the application of the PFIC rules to us.
Default PFIC Rules Under Section 1291 of the Code.
Generally, if we are or have been treated as a PFIC for any taxable year during a U.S. Holder’s holding period of common shares, subject to the special rules described below applicable to a U.S. Holder who makes a Mark-to-Market Election or a QEF Election (each as defined below), any “excess distribution” with respect to the common shares would be allocated ratably over the U.S. Holder’s holding period. The amounts allocated to the taxable year of the excess distribution and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations in that taxable year, as appropriate, and an interest charge would be imposed on the amount allocated to that taxable year. Distributions made in respect of common shares during a taxable year will be excess distributions to the extent they exceed 125% of the average of the annual distributions on common shares received by the U.S. Holder during the preceding three taxable years or the U.S. Holder’s holding period, whichever is shorter. In addition, dividends generally will not be qualified dividend income if we are a PFIC in the taxable year of payment or the preceding year.
Generally, if we are treated as a PFIC for any taxable year during which a U.S. Holder owns common shares, any gain on the disposition of the common shares would be treated as an excess distribution and would be allocated ratably over the U.S. Holder’s holding period and subject to taxation in the same manner as described in the preceding paragraph, and would not be eligible for the preferential long-term capital gains rate.
Certain elections (including the Mark-to-Market Election and the QEF Election, as defined and discussed below) may sometimes be used to mitigate the adverse impact of the PFIC rules on U.S. Holders, but these elections may accelerate the recognition of taxable income and have other adverse results.
Each current or prospective U.S. Holder should consult its own tax advisor regarding potential status of us as a PFIC, the possible effect of the PFIC rules to such holder in their particular circumstances, information reporting required if we were treated as a PFIC and the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
QEF Election.
A U.S. Holder of common shares in a PFIC generally would not be subject to the PFIC rules discussed above if the U.S. Holder had made a timely and effective election (a “QEF Election”) to treat us as a “qualified electing fund” (a “QEF”). Instead, such U.S. Holder would be subject to U.S. federal income tax on its pro rata share of our (i) net capital gain, which would be taxed as long-term capital gain to such U.S. Holder, and (ii) ordinary earnings, which would be taxed as ordinary income to such U.S. Holder, in each case regardless of whether such amounts are actually distributed to such U.S. Holder. However, a U.S. Holder that makes a QEF Election may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as “personal interest,” which is not deductible.
| 63 |
A U.S. Holder that makes a timely and effective QEF Election generally (a) may receive a tax-free distribution from us to the extent that such distribution represents our “earnings and profits” that were previously included in income by such U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the common shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, for U.S. federal income tax purposes, a U.S. Holder that makes a timely QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of the common shares.
A QEF Election will be treated as “timely” if such QEF Election is made for the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such first year. If a U.S. Holder makes a QEF Election after the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC, then, in addition to filing the QEF Election documents, a U.S. Holder may elect to recognize gain (which will be taxed under the rules discussed under “—Default PFIC Rules Under Section 1291 of the Code”) as if the common shares were sold on the qualification date. The “qualification date” is the first day of the first taxable year in which we are a QEF with respect to such U.S. Holder. The election to recognize such gain can only be made if such U.S. Holder’s holding period for the common shares includes the qualification date. By electing to recognize such gain, such U.S. Holder will be deemed to have made a timely QEF Election. In addition, under very limited circumstances, it is possible that a U.S. Holder might make a retroactive QEF Election if such U.S. Holder failed to file the QEF Election documents in a timely manner. If a U.S. Holder fails to make a QEF Election for the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC and does not elect to recognize gain as if the common shares were sold on the qualification date, such holder will not be treated as having made a “timely” QEF Election and will continue to be subject to the special adverse taxation rules discussed above under “—Default PFIC Rules Under Section 1291 of the Code”.
A QEF Election will apply to the taxable year for which such QEF Election is made and to all subsequent taxable years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent taxable year, we cease to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those taxable years in which we are not a PFIC. Accordingly, if we become a PFIC in another subsequent taxable year, the QEF Election will be effective and the U.S. Holder will be subject to the rules described above during any such subsequent taxable year in which we qualify as a PFIC.
A U.S. Holder cannot make and maintain a valid QEF Election unless we provide certain U.S. tax information necessary to make such an election. On an annual basis, we intend to use commercially reasonable efforts to make available to U.S. Holders, upon their written request (a) timely information as to our status as a PFIC, and (b) for each year in which we are a PFIC, information and documentation that a U.S. Holder making a QEF Election with respect to us is required to obtain for U.S. federal income tax purposes. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a QEF Election with respect to us.
Mark-to-Market Election.
A U.S. Holder of common shares in a PFIC would not be subject to the PFIC rules discussed above under “—Default PFIC Rules Under Section 1291 of the Code” if the U.S. Holder had made a timely and effective election to mark the PFIC common shares to market (a “Mark-to-Market Election”).
A U.S. Holder may make a Mark-to-Market Election with respect to the common shares only if such shares are marketable stock. Such shares generally will be “marketable stock” if they are regularly traded on a “qualified exchange,” which is defined as (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to section 11A of the Exchange Act, or (c) a non-U.S. securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such non-U.S. exchange has trading volume, listing, financial disclosure, surveillance, and other requirements, and the laws of the country in which such non-U.S. exchange is located, together with the rules of such non-U.S. exchange, ensure that such requirements are actually enforced and (ii) the rules of such non-U.S. exchange ensure active trading of listed stocks. Our common shares will generally be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of common shares is traded on a qualified exchange for at least 15 days during each calendar quarter. Each U.S. Holder should consult its own tax advisor with respect to the availability of a Mark-to-Market Election with respect to the common shares.
| 64 |
In general, a U.S. Holder that makes a timely Mark-to-Market Election with respect to the common shares will include in ordinary income, for each taxable year in which we are a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the common shares as of the close of such taxable year over (b) such U.S. Holder’s tax basis in such shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the lesser of (a) the excess, if any, of (i) such U.S. Holder’s adjusted tax basis in the common shares over (ii) the fair market value of such shares as of the close of such taxable year or (b) the excess, if any, of (i) the amount included in ordinary income because of such Mark-to-Market Election for prior taxable years over (ii) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years. If a U.S. Holder makes a Mark-to-Market Election after the first taxable year in which we are a PFIC and such U.S. Holder has not made a timely QEF Election with respect to us, the PFIC rules described above under “—Default PFIC Rules Under Section 1291 of the Code” will apply to certain dispositions of, and distributions on, the common shares, and the U.S. Holder’s mark-to-market income for the year of the election. If we were to cease being a PFIC, a U.S. Holder that marked its common shares to market would not include mark-to-market gain or loss with respect to its common shares for any taxable year that we were not a PFIC.
A U.S. Holder that makes a Mark-to-Market Election generally will also adjust such U.S. Holder’s tax basis in his common shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of the common shares subject to a Mark-to-Market Election, any gain or loss on such disposition will be ordinary income or loss (to the extent that such loss does not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior taxable years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years). A Mark-to-Market Election applies to the taxable year in which such Mark-to-Market Election is made and to each subsequent taxable year, unless the common shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a Mark-to-Market Election with respect to the common shares.
Reporting.
If we were to be treated as a PFIC in any taxable year, a U.S. Holder will generally be required to file an annual report with the IRS containing such information as the U.S. Treasury Department may require.
Each U.S. Holder should consult its own tax advisor regarding our potential status as a PFIC, the possible effect of the PFIC rules to such holder and information reporting required if we were a PFIC, as well as the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
Receipt of Foreign Currency
The amount of a distribution paid in Canadian dollars or Canadian dollar proceeds received on the sale or other taxable disposition of common shares will generally be equal to the U.S. dollar value of the currency on the date of receipt. If any Canadian dollars received with respect to the common shares are later converted into U.S. dollars, U.S. Holders may realize foreign currency gain or loss on the conversion. Any gain or loss generally will be treated as ordinary income or loss and generally will be from sources within the United States for U.S. foreign tax credit purposes. Each U.S. Holder should consult its own tax advisor concerning the possibility of foreign currency gain or loss if any such currency is not converted into U.S. dollars on the date of receipt.
Foreign Tax Credit
Subject to certain limitations, a U.S. Holder who pays (whether directly or through withholding) Canadian or other non-U.S. income tax with respect to the common shares may be entitled, at the election of the U.S. Holder, to receive either a deduction or a credit for Canadian or other non-U.S. income tax paid. Dividends paid on common shares generally will constitute income from sources outside the United States. Any gain from the sale or other taxable disposition of the common shares by a U.S. Holder generally will constitute U.S. source income. The foreign tax credit rules (including the limitations with respect thereto) are complex, and each U.S. Holder should consult its own tax advisor regarding the foreign tax credit rules, having regard to such holder’s particular circumstances.
| 65 |
Information Reporting; Backup Withholding
Generally, information reporting and backup withholding will apply to distributions on, and the payment of proceeds from the sale or other taxable disposition of, the common shares unless (i) the U.S. Holder is a corporation or other exempt entity, or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number, certifies that the U.S. Holder is not subject to backup withholding and otherwise complies with the applicable requirements of the backup withholding rules.
Backup withholding is not an additional tax. Any amount withheld generally will be creditable against a U.S. Holder’s U.S. federal income tax liability or refundable to the extent that it exceeds such liability provided the required information is provided to the IRS in a timely manner.
In addition, certain categories of U.S. Holders must file information returns with respect to their investment in a non-U.S. corporation. For example, certain U.S. Holders must file IRS Form 8938 with respect to certain “specified foreign financial assets” (such as the common shares) with an aggregate value in excess of US$50,000 (and, in some circumstances, a higher threshold). Failure to do so could result in substantial penalties and in the extension of the statute of limitations with respect to such holder’s U.S. federal income tax returns. Each U.S. Holder should consult its own tax advisor regarding application of the information reporting and backup withholding rules to it in connection with an investment in our common shares.
Medicare Contribution Tax
U.S. Holders that are individuals, estates or certain trusts generally will be subject to a 3.8% Medicare contribution tax on, among other things, dividends on, and capital gains from the sale or other taxable disposition of, common shares, subject to certain limitations and exceptions. Each U.S. Holder should consult its own tax advisor regarding possible application of this additional tax to income earned in connection with an investment in our common shares.
Recent Sales of Unregistered Securities
None.
Issuer Repurchases of Equity Securities
None.
| Item 6. | Selected Financial Data |
Not applicable.
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operation |
The following discussion should be read in conjunction with the attached consolidated financial statements and notes thereto. This annual report contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those expressed or implied by such forward-looking statements. For a detailed discussion of these risks and uncertainties, see Item 1A, “Risk Factors” of this annual report. We caution the reader not to place undue reliance on these forward-looking statements, which reflect management’s analysis only as of the date of this annual report. We undertake no obligation to update forward-looking statements which reflect events or circumstances occurring after the date of this annual report, unless required by applicable securities laws.
| 66 |
Introduction
This management’s discussion and analysis, or MD&A, is presented in order to provide the reader with an overview of the financial results and changes to our financial position as at March 31, 2020 and for the three and twelve-month periods then ended. This MD&A explains the material variations in our financial statements of operations, financial position and cash flows for the three and twelve-month periods ended March 31, 2020, and 2019.
Market data and certain industry data and forecasts included in this MD&A were obtained from internal corporation surveys, market research, and publicly available information, reports of governmental agencies and industry publications and surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of that information is not guaranteed. We have not independently verified any of the data from third-party sources or the underlying economic assumptions they made. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s knowledge of our industry, have not been independently verified. Our estimates involve risks and uncertainties, including assumptions that may prove not to be accurate, and these estimates and certain industry data are subject to change based on various factors, including those discussed under Item 1.A “Risk Factors” in this annual report. While we believe our internal business research is reliable and the market definitions we use in this MD&A are appropriate, neither our business research nor the definitions we use have been verified by any independent source. This MD&A may only be used for the purpose for which it has been published.
This MD&A, approved by the Board of Directors on June 29, 2020, should be read in conjunction with our audited consolidated financial statements for the year ended March 31, 2020 and 2019. Our audited financial statements were prepared in accordance with generally accepted accounting principles issued by the Financial Accounting Standards Board in the United States, or GAAP. Up to and including the third quarter ended December 31, 2019, we prepared our consolidated financial statements in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board. The comparative information in our financial statements for the year ended March 31, 2020 has been adjusted, as necessary, to be compliant with our accounting policies under GAAP. Our financial results are now published in United States dollars. Effective March 31, 2020, the reporting currency used in the consolidated financial statements has changed from Canadian dollars to U.S. dollars. This change in reporting currency has been applied in the financial statements retrospectively such that all amounts expressed in our consolidated financial statements and the accompanying notes thereto are in U.S. dollars. All amounts appearing in this MD&A for the period by period discussions are in thousands of U.S. dollars, except share and per share amounts or unless otherwise indicated.
COVID-19 Update
To date, the ongoing COVID-19 pandemic has not caused significant disruptions to our business operations and research and development activities. In January 2020, before the COVID-19 pandemic started to have a widespread impact in North America, the last patients completed their final visits to our TRILOGY Phase 3 trials. However, in light of our plan to raise additional capital (dilutive or non-dilutive) to fully execute our business plan, a continuation of the COVID-19 pandemic and any resulting volatility generally in the capital markets could adversely impact our ability to access capital on terms acceptable to us or at all. In addition, a continuation of the COVID-19 pandemic in North America could negatively affect our ability to conduct additional clinical work, if we require any. See “Item 1A, Risk Factors – General Risks Related to the Company – Our business and operations may be materially and adversely affected by the recent COVID-19 pandemic ..”
| 67 |
Caution Regarding Non-GAAP Financial Measures
We use multiple financial measures for the review of our operating performance. These measures are generally GAAP financial measures, but one adjusted financial measure, non-GAAP operating loss, is also used to assess our operating performance. This non-GAAP financial measure is directly derived from our financial statements and is presented in a consistent manner. We use this measure, in addition to the GAAP financial measures, for the purposes of evaluating our historical and prospective financial performance, as well as our performance relative to competitors and to plan and forecast future periods as well as to make operational and strategic decisions. We believe that providing this non-GAAP information to investors, in addition to GAAP measures, allows them to see our results through the eyes of management, and to better understand our historical and future financial performance.
Earnings and other measures adjusted to a basis other than GAAP do not have standardized meanings and are unlikely to be comparable to similar measures used by other companies. Accordingly, they should not be considered in isolation. We use non-GAAP operating loss to measure our performance from one period to the next without the variation caused by certain adjustments that could potentially distort the analysis of trends in our operating performance, and because we believe it provides meaningful information on our financial condition and operating results. Our method for calculating non-GAAP operating loss may differ from that used by other companies.
We calculate our non-GAAP operating loss by adding to net loss our finance expenses (which includes change in fair value of derivative warrant liabilities, foreign exchange gain (loss), interest expense and accretion on convertible debentures, and transaction costs related to derivative warrant liabilities, net of interest income) depreciation and amortization, impairment loss, litigation settlement that was settled via the issuance of common shares, and stock-based compensation, and by subtracting deferred tax recovery. Items that do not impact our core operating performance are excluded from the calculation as they may vary significantly from one period to another. We also exclude the effects of certain non-monetary transactions recorded, such as stock-based compensation and litigation settlement that was settled via the issuance common shares, from our non-GAAP operating loss calculation. Excluding these items does not imply they are necessarily non-recurring.
A reconciliation of net loss to non-GAAP operating loss is presented later in this MD&A.
| 68 |
Basis of presentation of the financial statements
Our consolidated financial statements, which include the accounts of our subsidiary AIAG, have been prepared in accordance with GAAP and the rules and regulations of the SEC related to annual reports filed on Form 10-K. All intercompany transactions and balances are eliminated on consolidation.
Going concern uncertainty
The following summarizes the principal conditions or events relevant to our going concern assessment, which primarily considers the period of one year from the issuance date of our consolidated financial statements. We have incurred operating losses and negative cash flows from operations since our inception. Our current assets of $16.1 million as at March 31, 2020 include cash and cash equivalents totaling $14.2 million. Our current liabilities total $7.4 million at March 31, 2020 and are comprised primarily of amounts due to or accrued for creditors. Management projects that assuming positive results from our TRILOGY Phase 3 program, additional funds will be needed in the future for us to file an NDA to obtain FDA approval for CaPre in the United States, to further scale-up our manufacturing capabilities, and to complete marketing and other pre-commercialization activities. Our plans include raising additional capital through additional securities offerings, as well as non-dilutive sources of capital such as grants or loans and strategic alliances, but there can be no assurance as to when or whether we will complete any financings or strategic alliances. In particular, raising additional equity capital is subject to market conditions not within our control. If we do not raise additional funds or find one or more strategic partners, we may not be able to realize our assets and discharge our liabilities in the normal course of business. We have no arranged sources of financing currently other than our “At-the-Market” sales agreement which provides for only conditional selling of our common shares.
As a result, there is a substantial doubt about our ability to continue as a going concern. Our consolidated financial statements have been prepared on a going concern basis, which assumes we will continue our operations in the foreseeable future and will be able to realize our assets and discharge our liabilities and commitments in the ordinary course of business. These consolidated financial statements do not include any adjustments to the carrying values and classification of assets and liabilities and reported expenses that might result from the outcome of this uncertainty and that may be necessary if the going concern basis was not appropriate for these consolidated financial statements. If we were unable to continue as a going concern, material impairment of to the carrying values of our assets, including the intangible asset, could be required.
| 69 |
Comparative financial information for the three-month periods and years ended March 31, 2020 and 2019
| Three-month periods ended | Year ended | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net income (loss) | 16,615 | (12,690 | ) | (25,513 | ) | (39,366 | ) | |||||||||
| Basic and diluted gain (loss) per share | 0.18 | (0,16 | ) | (0.30 | ) | (0.73 | ) | |||||||||
| Non-GAAP operating (loss)1 | (2,986 | ) | (9,092 | ) | (22,315 | ) | (30,555 | ) | ||||||||
| Total assets | 22,853 | 36,896 | 22,853 | 36,896 | ||||||||||||
| Working capital2 | 8,684 | 14,296 | 8,684 | 14,296 | ||||||||||||
| Total non-current financial liabilities | 2,464 | 12,183 | 2,464 | 12,183 | ||||||||||||
| Total shareholders’ equity | 12,994 | 11,045 | 12,994 | 11,045 | ||||||||||||
Reconciliation of net loss to non-GAAP Operating Loss
| Three-month periods ended | Year ended | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net income (loss) | 16,615 | (12,690 | ) | (25,513 | ) | (39,366 | ) | |||||||||
| Add (deduct): | ||||||||||||||||
| Stock-based compensation | 445 | 107 | 1,953 | 777 | ||||||||||||
| Depreciation and amortization | 600 | 595 | 2,319 | 2,334 | ||||||||||||
| Common shares issued as a legal settlement | – | 741 | – | 741 | ||||||||||||
| Financial (income) expenses | (20,646 | ) | 2,155 | (1,075 | ) | 4,959 | ||||||||||
| Non-GAAP operating gain (loss) | (2,986 | ) | (9,092 | ) | (22,315 | ) | (30,555 | ) | ||||||||
Results of operations for the three and twelve-month periods ended March 31, 2020 and 2019
Three months ended March 31, 2020 and 2019
The net income of $16,615 or $0.18 per share for the three months ended March 31, 2020 increased by $29,305 from the net loss $12,690 or ($0.16) per share for the three months ended March 31, 2019.
The net income resulted primarily from a net financial gain of $20,646 for the three months ended March 31, 2020, as compared to net financial expense of $2,155 for the three months ended March 31, 2019, due mostly to the change in fair value of the warrant derivative liability, which decreases as our share price decreases, partially offset by a decrease in the number of warrants outstanding due to exercises in the current period. In addition, the gain was also due to the decrease in research and development expenses of $6,243 as the TRILOGY Phase 3 clinical program for CaPre moved closer to completion.
The financial gain was partially offset by increased sales and marketing expenses of $298 due to increased headcount to support expanded business development activities, and by additional accounting and legal fees incurred in connection with the conversion of the financial statements from IFRS to GAAP, as well as higher insurance cost. Stock-based compensation expense increased by $338 as result of 6.1 million stock options granted to existing and new employees and directors during year ended March 31, 2020, partially offset by stock options exercised, forfeited and expired. The weighted average fair value of the options granted to employees and directors during the year ended March 31, 2020 was CAD$0.85 compared to CAD$0.51 for the year ended March 31, 2019 grants.
_____________________________
| 1 | The Non-GAAP operating loss is not a standard measure endorsed by GAAP requirements. A reconciliation to our net loss is presented in this MD&A. |
| 2 | Working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by GAAP requirements, the results may not be comparable to similar measurements presented by other public companies. |
| 70 |
Years ended March 31, 2020, and 2019
The net loss of $25,513 or ($0.30) per share for the year March 31, 2020 decreased by $13,853 from the net loss for the year ended March 31, 2019 of $39,366 or ($0.73) per share. The per share loss decreased in line with the lower net loss and with the issuance of shares in relation mainly to the public financings that occurred in May and October 2018, the exercise of warrants during July and August 2019 and the sale of shares under the at-the-market program during the second half of fiscal year 2020.
The decreased net loss was primarily due to a reduction of research and development expenses of $13,399, as the TRILOGY Phase 3 clinical program for CaPre moved closer to completion. In addition, the decrease in net loss resulted from lower net financial expenses of $1,075 for the year ended March 31, 2020, as compared to net financial expenses of $4,960 for the year ended March 31, 2019, due mostly to the change in fair value of the warrant derivative liability, partially offset by a decrease in the number of warrants.
In contrast, sales and marketing expenses increased by $2,171 due to the increase in headcount to support expanded business and market development activities, and additional administrative fees were incurred in connection with the implementation of a new enterprise resources planning system, and increased insurance cost, as well as increased accounting and legal fees associated with the conversion from IFRS to GAAP.
Furthermore, stock-based compensation expense increased by $1,176 as result of 6.1 million stock options granted to existing and new employees and directors during the year ended March 31, 2020, partially offset by stock options exercised, forfeited and expired. The weighted average fair value of the options granted to employees and directors during the year ended March 31, 2020 was CAD$0.85 compared to CAD$0.51 for the year ended March 31, 2019.
| 71 |
Breakdown of major components of the statement of loss and comprehensive loss
| Research and development expenses | ||||||||||||||||
| Three Months Ended | Year Ended | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits | 476 | 508 | 1,759 | 1,374 | ||||||||||||
| Research contracts | 669 | 6,775 | 10,260 | 24,676 | ||||||||||||
| Professional fees | 119 | 363 | 1,117 | 925 | ||||||||||||
| Other | 81 | 94 | 392 | 331 | ||||||||||||
| Government grants & tax credits | (117 | ) | (223 | ) | (313 | ) | (445 | ) | ||||||||
| Sub-total | 1,228 | 7,517 | 13,215 | 26,861 | ||||||||||||
| Stock-based compensation | 93 | 50 | 443 | 184 | ||||||||||||
| Depreciation and amortization | 597 | 594 | 2,316 | 2,328 | ||||||||||||
| Total | 1,918 | 8,161 | 15,974 | 29,373 | ||||||||||||
| General and administrative expenses | ||||||||||||||||
| Three Months Ended | Year Ended | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits | 385 | 601 | 1,506 | 1,490 | ||||||||||||
| Professional fees | 615 | 456 | 2,018 | 1,193 | ||||||||||||
| Other | 291 | 277 | 1,058 | 593 | ||||||||||||
| Sub-total | 1,291 | 1,334 | 4,582 | 3,276 | ||||||||||||
| Stock-based compensation | 258 | 34 | 1,217 | 522 | ||||||||||||
| Legal settlement expected to be settled via common shares | – | 741 | – | 741 | ||||||||||||
| Total | 1,549 | 2,109 | 5,799 | 4,539 | ||||||||||||
| Sales and Marketing Expenses | ||||||||||||||||
| Three Months Ended | Year Ended | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits | 389 | 102 | 1,206 | 261 | ||||||||||||
| Professional fees | 48 | 133 | 711 | 147 | ||||||||||||
| Other | 32 | 8 | 455 | 15 | ||||||||||||
| Sub-total | 469 | 243 | 2,372 | 423 | ||||||||||||
| Stock-based compensation | 94 | 22 | 293 | 71 | ||||||||||||
| Total | 563 | 265 | 2,665 | 494 | ||||||||||||
| 72 |
Three months ended March 31, 2020 compared to the three months ended March 31, 2019
During the three months ended March 31, 2020, we continued our advancement of the two-study TRILOGY Phase 3 clinical program for CaPre, in partnership with one of the world’s largest providers of biopharmaceutical development and clinical outsourcing services. Research and development expenses before depreciation, amortization and stock-based compensation expense for the three months ended March 31, 2020 totaled $1,228 compared to $7,517 for the three months ended March 31, 2019. This $6,289 net decrease was mainly attributable to a $6,106 decrease in research contracts, and $244 decrease in professional fees. The lower research contract expense is attributed primarily to the advancement of the Phase 3 clinical trial program, as it moved closer to completion.
General and administrative expenses totaled $1,291 before stock-based compensation expense for the three months ended March 31, 2020 and decreased by $43 from $1,334 for the three months ended March 31, 2019. The decrease is mainly attributable to the timing of recognition of bonus expense, partially offset by increased professional accounting and legal fees in connection with the conversion from IFRS to U.S. GAAP.
Sales and marketing expenses were $469 before stock-based compensation expense for the three months ended March 31, 2020 compared to $243 for the three months ended March 31, 2019. The increase is in line with a higher headcount in the commercial team to support expanded business and market development activities. The increase was partially offset by a reduction in professional fees as a result of a slowdown in pre-launch marketing activities until the results of the TRILOGY Phase 3 clinical studies are obtained.
The stock-based compensation expense increased by $339 to $445 for the three months ended March 31, 2020 from $106 for the three months ended March 31, 2019. The increase is mainly due to 6.1 million stock options granted to existing and new employees and directors during the year ended March 31, 2020, partially offset by stock options exercised, forfeited and expired. The weighted average fair value of the options granted to employees and directors during the year ended March 31, 2020 was CAD$0.85, compared to CAD$0.51 for the year ended March 31, 2019.
The depreciation and amortization expense remained relatively constant.
Financial income for the three months ended March 31, 2020 was $20,646 compared to a financial expense of $2,154 for the three months ended March 31, 2019. The net increase in financial income of $22,800 was mainly attributable to $21,817 gain from the changes in fair value of derivative warrant liabilities, partially offset by a lower number of warrants.
Year ended March 31, 2020 compared to year ended March 31, 2019
During the year ended March 31, 2020, we continued our advancement of the two-study TRILOGY Phase 3 clinical program for CaPre. Research and development expenses before depreciation, amortization and stock-based compensation expense for the year ended March 31, 2020 totaled $13,215, compared to $26,861 for the year ended March 31, 2019. This $13,646 net decrease was mainly attributable to a $14,416 decrease in research contracts, partially offset by an increase in salaries and benefits of $385 due to increased headcount and related benefits. The lower research contract expense is attributed primarily to the advancement of the Phase 3 clinical trial program moved closer to completion.
General and administrative expenses totaled $4,582 before stock-based compensation expense for the year ended March 31, 2020 and increased by $1,306 from $3,276 for the year ended March 31, 2019. This increase was mainly attributable to a $446 increase associated with our insurance policy, as well as an increase of $829 in accounting, corporate and legal fees.
Sales and marketing expenses were $2,372 before stock-based compensation expense for the year ended March 31, 2020 compared to $423 for the year ended March 31, 2019. The increase is in line with a higher headcount in the commercial team to support expanded business and market development activities.
The stock-based compensation expense increased by $1,176 to $1,953 for the year ended March 31, 2020 from $777 for the year ended March 31, 2019. The increase is mainly due to 6.1 million stock options granted to existing and new employees and directors during the year ended March 31, 2020, partially offset by stock options exercised, forfeited and expired. The weighted average fair value of the options granted to employees and directors during the year ended March 31, 2020 was CAD$0.85, compared to CAD$0.51 for the year ended March 31, 2019.
| 73 |
The depreciation and amortization expense remained constant.
Net financial expenses for the year ended March 31, 2020 was $1,075 compared to net financial expenses of $4,960 for the year ended March 31, 2019. The net decrease in loss of $3,885 was mainly attributable to a decrease in the fair value of derivative warrant liabilities of $2,663, partially offset by a decrease in financing transaction costs and a lower number of warrants outstanding that are classified as a liability and subject to remeasurement.
Two separate derivative warrant liabilities are included in the statement of financial position as at March 31, 2020, and March 31, 2019. These derivative warrant liabilities stem from the financing transactions that took place in May 2018 and December 2017. The derivative warrant liabilities are re-measured to fair value at each reporting date using the Black-Scholes option pricing model. The valuations are mainly driven by the fluctuation in our share price resulting in an increased or decreased loss or gain related to the change in fair value of the warrant liabilities and increasing or decreasing the corresponding liability in the statement of financial position.
Liquidity and Capital Resources
Share Capital Structure
Our authorized share capital consists of an unlimited number of Class A, Class B, Class C, Class D and Class E shares, without par value. Issued and outstanding fully paid shares, stock options, restricted shares units and warrants, were as follows for the periods ended:
| March 31, 2020 | March 31, 2019 | |||||||
| Number outstanding | Number outstanding | |||||||
| Class A shares, voting, participating and without par value | 90,209,449 | 78,132,734 | ||||||
| Stock options granted and outstanding | 9,936,486 | 4,046,677 | ||||||
| May 2018 public offering of warrants exercisable at CAD$1.31, until May 9, 2023 | 6,593,750 | 10,188,100 | ||||||
| Public offering broker warrants May 2018 exercisable at CAD$1.05 until May 9, 2023 | 222,976 | 547,975 | ||||||
| December 2017 U.S. public offering of warrants exercisable at US$1.26, until December 19, 2022 | 7,072,962 | 9,801,861 | ||||||
| December 2017 U.S. broker warrants exercisable at US$1.2625, until December 27, 2022 | 259,121 | 495,050 | ||||||
| February 2017 public offering of warrants exercisable at CAD$2.15, until February 21, 2022 | 1,723,934 | 1,904,034 | ||||||
| 2017 unsecured convertible debentures conversion option contingent warrants exercisable at $1.90, until February 21, 20203 | – | 1,052,630 | ||||||
| Total fully diluted shares | 116,018,678 | 106,169,061 |
___________________________
| 3 | The debentures were convertible into common shares at a fixed price of CAD$1.90 per common share except if we pay, before the maturity, all or any portion of the convertible debentures. We paid the total balance of the debenture in cash at the maturity date. |
| 74 |
Cash Flows and Financial Condition between the years ended March 31, 2020 and 2019
Summary
As at March 31, 2020, cash and cash equivalents totaled $14,240, a net decrease of $2,631 compared to cash and cash equivalents totaling $16,871 at March 31, 2019.
Operating activities
During the years ended March 31, 2020 and March 31, 2019, our operating activities used cash of $22,944 and $24,787, respectively. The decrease of $1,843 during the year ended March 31, 2020, was due to the reduction of spend as the TRILOGY Phase 3 clinical trials were nearing completion, partly offset by the timing of payment of invoices.
We expect that additional time and capital will be required by us to file an NDA to obtain FDA approval for CaPre in the United States, to further scale-up our manufacturing capabilities, and to complete marketing and other pre-commercialization activities, if our TRILOGY Phase 3 program is successful and we can proceed to file an NDA. Consequently, we expect to require additional capital to fund our daily operating needs beyond the next fiscal year-end. Based on a conservative estimate, we believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements through the first calendar quarter of 2021. To fully execute our business plan, we plan to raise the necessary capital primarily through additional securities offerings and multiple sources of non-dilutive capital such as grants or loans and strategic alliances. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay the commercial launch of CaPre. Negative or inconclusive results in our TRILOGY Phase 3 clinical program for CaPre may adversely affect our ability to raise additional capital and/or to complete strategic commercialization partnerships to support the commercial launch of CaPre. Additional funding from third parties may not be available on acceptable terms or at all to enable us to continue with the commercialization of CaPre.
Investing activities
During the year ended March 31, 2020, we generated cash of $8,138 due primarily to the maturity of marketable securities.
During the year ended March 31, 2019, we used cash of $9,442 due primarily to the acquisition of marketable securities.
Financing activities
During the year ended March 31, 2020, we generated cash of $13,176 due primarily to the net proceeds from the sale of shares under the “at-the-market”, or ATM, program for a total of $6,981 and the exercise of warrants for a total of $7,706, partially offset by the payment of convertible debentures upon their maturity for a total of $1,556.
During the year ended March 31, 2019, our financing activities generated cash of $45,690 mainly from the net proceeds of the public offerings of $44,892 and proceeds from warrants of $796.
ATM Program
On February 14, 2019, we entered into an ATM sales agreement with B. Riley FBR, Inc., pursuant to which common shares may be sold from time to time for aggregate gross proceeds of up to $30 million, with sales only being made on the NASDAQ Stock Market. The common shares may be distributed at market prices prevailing at the time of any sale and as a result, prices may vary between purchasers and during the period of distribution. During the year ended March 31, 2020, a total of 4.1 million common shares were sold for total net proceeds of approximately $6.9 million under the ATM program. The shares were sold at the prevailing market prices, which resulted in an average price of approximately $1.79 per share. As at March 31, 2020, costs incurred in connection to the ATM amounted to $217 and were recorded as deferred financing while proportional costs related to the common shares sold for a total of $40 were reclassified to equity.
| 75 |
There are several conditions that must be met in order for us to access the ATM and the program only commits the agent to use commercially reasonable efforts, and thus is not a guaranteed source of financing. Further, the ATM may be cancelled by the agent at its sole discretion at any time with 5 days’ notice. In the event we are unable to use our ATM, we would have to rely on other financing approaches and sources to obtain additional new funding.
Transactions Subsequent to March 31, 2020
Subsequent to March 31, 2020, we sold a total of 2,278,936 common shares through the ATM program, for net proceeds of approximately $1.8 million (net of commissions paid for approximately $0.08 million). The shares were sold at the prevailing market prices which resulted in an average price of approximately $0.81 per share.
October 2018 Public Offering
On October 9, 2018, we closed a U.S. public offering of 16,600,000 common shares at a price of $1.00 per share. In addition, the underwriters fully exercised their over-allotment option to purchase 2,490,000 additional common shares at the same public offering price. This offering generated gross proceeds of $19.1 million (CAD$24.7 million), which resulted in net proceeds to us of $17.4 million (CAD$22.6 million) and a total of 19,090,000 common shares issued.
On October 23, 2018, we closed a Canadian public offering of 18,750,000 common shares at a price of CAD$1.28 per share. In addition, the underwriters fully exercised their over-allotment option to purchase 2,812,500 additional common shares at the same public offering price. This offering generated gross proceeds of $21.1 million (CAD$27.6 million), which resulted in net proceeds to us of approximately $19.4 million (CAD$25.4 million) and a total of 21,562,500 common shares issued.
May 2018 Public Offering
On May 9, 2018, we closed a Canadian public offering of 9,530,000 units at a price of CAD$1.05 per unit for gross proceeds of $7.8 million (CAD$10 million). The units issued consist of 9,530,000 common shares and 9,530,000 warrants. Each warrant entitles the holder thereof to acquire one common share at an exercise price of CAD$1.31 at any time until May 9, 2023.
On May 14, 2018, the underwriters exercised their over-allotment option by purchasing an additional 1,429,500 units at a price of CAD$1.05 per unit, for additional gross proceeds of $1.1 million (CAD$1.5 million). The over-allotment units issued consist of 1,429,500 common shares and 1,429,500 warrants. Each Warrant entitles the holder thereof to acquire one common share at an exercise price of CAD$1.31 at any time until May 9, 2023.
At the time of issuance, the warrant component of these units are derivative warrant liabilities for accounting purposes due to certain contingent provisions that allow for cash settlement in the warrant agreement (see note 13 of our consolidated financial statements). The proceeds of the offering are required to be split between the derivative warrant liabilities and the equity-classified Common shares at the time of issuance of the units. The fair value of the derivative warrant liabilities at the time of issuance was determined to be $3.3 million (CAD$4.3 million) and the residual of the proceeds of $4.8 million (CAD$6.2 million) were allocated to the common shares. Issuance costs related to this transaction totaled approximately $1.4 million (CAD$1.8 million) and have been allocated between the derivative warrant liabilities and common shares based on relative value. Resulting from this allocation, $0.5 million (CAD$0.7 million) has been allocated to the derivative warrant liabilities and is recognized in finance expenses in the Statements of Earnings and Comprehensive Loss, whereas the remaining portion of $0.86 million (CAD$1.1 million) in issuance costs was allocated to the common shares and recognized as a reduction to common shares in the Balance Sheet.
| 76 |
The weighted average fair value of the public offering warrants issued in May 2018 was determined to be $0.30 (CAD$0.39) per warrant. Changes in the subsequent measurement of fair value of the warrants are recognized in financial expenses.
As part of the transaction, we also issued broker warrants to purchase up to 547,975 common shares. Each broker warrant entitles the holder thereof to acquire one common share at an exercise price of CAD$1.05, at any time until May 9, 2023. The broker warrants are considered to be equity-classified non-employee stock-based awards and are accounted for at fair value at grant date and not subsequently revalued.
Financial Position
The following table details the significant changes to the statements of financial position as at March 31, 2020 compared to the prior fiscal year end at March 31, 2019:
| Accounts | Increase (Decrease) $ | Comments | ||||
| Cash and cash equivalents | (2,631 | ) | See cash flow statement | |||
| Marketable securities | (8,908 | ) | Progression of research contracts | |||
| Receivables | (643 | ) | Mostly due to tax credit reimbursement from FY2019 and FY2018, partially offset by FY 2020 tax credit estimate | |||
| Deferred financing costs | 44 | Additional accounting and legal fees incurred in connection with the ATM program, net of costs applied to equity | ||||
| Prepaid expenses | 142 | Advances to vendors, including insurance policy, net of usage costs | ||||
| Equipment | (197 | ) | Acquisition of equipment net of depreciation | |||
| Right of use asset | 147 | Lease contract for Sherbrooke | ||||
| Intangible assets | (2,142 | ) | Amortization | |||
| Trade and other payables | (4,988 | ) | Timing of payments net of accruals and settlement of provision for legal settlement via the issuance of common shares | |||
| Derivative warrant liabilities | (9,790 | ) | Change in fair value and exercise of derivative warrants | |||
| Unsecured convertible debentures | (1,361 | ) | Cash payment at maturity date | |||
| Lease liability | (147 | ) | Lease contract for Sherbrooke | |||
See the statement of changes in equity in our financial statements for details of changes to the equity accounts since March 31, 2019.
Treasury Operations
Our treasury policy is to invest cash that is not required immediately into instruments with an investment strategy based on capital preservation. Cash equivalents and marketable securities are primarily made in guaranteed investment certificates, term deposits and high-interest savings accounts, which are issued and held with Canadian chartered banks, highly rated promissory notes issued by government bodies and commercial paper. We hold cash denominated in both U.S. and CAD dollars. Funds received in U.S. dollars from equity financings are invested as per our treasury policy in U.S. dollar investments and converted to CAD dollars as appropriate to fulfill operational requirements and funding.
Derivative warrant liabilities
The 10,188,100 warrants issued as part of our May 2018 public offering in Canada were recognized as derivative warrant liabilities with a fair value of $3,323. During the year ended March 31, 2020, a total of 3,594,350 warrants were exercised. As of March 31, 2020, the derivative warrant liability for the remaining 6,593,750 warrants totaled $1,146, which represents the fair value of these warrants. The weighted average fair value of the warrants issued in the May 2018 public offering in Canada was determined to be CAD$0.39 per warrant at inception and approximately CAD$0.24 (USD $0.17) per warrant as at March 31, 2020.
| 77 |
On December 27, 2017, 9,801,861 warrants were issued as part of our U.S. public offering and recognized as derivative warrant liabilities with a fair value of $4,548. The December 2017 warrants are derivative warrant liabilities for accounting purposes due to the currency of the exercise price (US$) being different from our Canadian dollar functional currency. During the year ended March 31, 2020, 2,728,899 warrants were exercised (including 52,288 warrants exercised on a cashless basis). As of March 31, 2020, the derivative warrant liability for the remaining 7,072,962 warrants totaled $1,247, which represents the fair value of these warrants. The weighted average fair value of the 2017 warrants issued was determined to be CAD$0.60 per warrant at inception and approximately CAD$0.25 (USD $0.17) per warrant as at March 31, 2020.
The decrease in the fair value of both existing derivative warrant liabilities as at March 31, 2020 is due to the decrease in our share price and the dilution factor.
During the year ended March 31, 2020, the following warrants were exercised with the resulting cash proceeds:
| Number exercised | Proceeds | |||||||
| $ | ||||||||
| May 2018 over-allotment warrants 2018 | 3,594,350 | 3,567 | ||||||
| December 2017 US public offering warrants 2017 | 2,676,611 | 3,373 | ||||||
| Canadian public offering warrants February 2017 | 180,100 | 292 | ||||||
| Canadian public offering broker warrants May 2018 | 325,000 | 257 | ||||||
| Contingent warrants private placement 2017 | 150,000 | 217 | ||||||
| 6,926,061 | 7,706 |
In addition, 235,929, broker warrants and 52,288 derivative warrants issued as part of the December 2017 U.S. public offering were exercised on a cashless basis to acquire 136,013 common shares.
During the year ended March 31, 2019, 771,400 warrants issued as part of the May 2018 Canadian public offering were exercised at an exercise price of CAD$1.31 per common share, resulting in $1.0 million of cash proceeds. In addition, 4,455 warrants issued as part of the December 2017 U.S. public offering were exercised on a cashless basis to acquire 1,074 common shares. A total of 772,474 common shares were issued as a result of 775,855 warrants being exercised.
| 78 |
Contractual Obligations and Commitments
As at March 31, 2020, our liabilities totaled $9,859, of which $7,395 was due within 1 year, and $2,393 related to derivative warrant liabilities that are expected to be settled in common shares.
A summary of the contractual obligations at March 31, 2020, is as follows:
| Contractual Obligations | Total | Less than 1 year | 1-3 years | More than 3 years | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Trade and other payables | 7,319 | 7,319 | – | – | ||||||||||||
| Operating lease obligations | 160 | 80 | 80 | – | ||||||||||||
| RKO supply agreement | 2,808 | 2,496 | 312 | – | ||||||||||||
| Total | 10,287 | 9,895 | 392 | – |
Lease
On March 5, 2020, we renewed the lease agreement for our research and development and quality control laboratory facility located in Sherbrooke, Québec, resulting in an obligation of $160 over 24 months of the lease term.
RKO supply agreement
On October 25, 2019, we signed a supply agreement with Aker, to purchase RKO for a committed volume of commercial starting material for CaPre at a fixed price for a total value of $3.1 million (take or pay). The delivery of the RKO has been established following a calendar year basis and it is expected to be completed in the 4th calendar quarter of 2021. As at March 31, 2020, the remaining balance of the commitment with Aker amounts to $2.8 million.
Research and development contracts and contract research organizations agreements
We utilize contract manufacturing organizations, for the development and production of clinical materials and contract research organizations to perform services related to our clinical trials. Pursuant to the agreements with these contract manufacturing organizations and contract research organizations, we have either the right to terminate the agreements without penalties or under certain penalty conditions.
Contingencies
We evaluate contingencies on an ongoing basis and establish loss provisions for matters in which losses are probable and the amount of the loss can be reasonably estimated.
On May 10, 2019, we announced the settlement regarding legal claims made by our former chief executive officer with respect to the termination of his employment. Pursuant to the settlement agreement, we agreed to issue 900,000 common shares valued at CAD$1.10 per share to the former CEO. In addition, we agreed to reimburse the former CEO for legal fees of $48. Pursuant to the settlement agreement, we received a full and final release from the former CEO on all procedures in connection with the termination of his employment. This settlement was accrued as a short-term liability as at March 31, 2019 and the expense of $790 was included as part of general and administrative expenses. The case is closed, and no further costs are expected.
Off-Balance Sheet Arrangements
As of the date of this annual report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
| 79 |
Use of estimates and measurement of uncertainty
The preparation of the financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities (see note 13 of the consolidated financial statements) and stock-based compensation (see note 15 of the consolidated financial statements). Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and developments expenditures at each reporting date, are determining which research and development expenses qualify for research and development tax credits and in what amounts. We recognize the tax credits once we have reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and therefore, could be different from the amounts recorded.
Critical Accounting Policies
Derivative warrant liabilities
The warrants forming part of the units issued in the May 2018 Canadian public offering are derivative liabilities for accounting purposes given the fact that the warrant indenture contains certain contingent provisions that allow for cash settlement. The warrants forming part of the units issued from the December 2017 U.S. public offering are derivative liabilities for accounting purposes due to the currency of the exercise price being different from our functional currency. The derivative warrant liabilities are required to be measured at fair value at each reporting date with changes in fair value recognized in earnings. We use the Black-Scholes pricing model to determine the fair value. The model requires the assumption of future stock price volatility, which is estimated based on weighted average historic volatility. Changes to the expected volatility could cause significant variations in the estimated fair value of the derivative warrant liabilities.
Stock-based compensation
We have a stock-based compensation plan, which is described in note 15 of the consolidated financial statements. We account for stock options granted to employees based on the fair value method, with fair value determined using the Black-Scholes model. The Black Scholes model requires certain assumptions such as future stock price volatility and expected life of the instrument. Expected volatility is estimated based on weighted average historic volatility. The expected life of the instrument is estimated based on the average of the vesting and contractual periods for employee awards as there is minimal prior exercises of options in which to establish historical exercise experience; and contractual life is used for broker warrants. Under the fair value method, compensation cost is measured at fair value at date of grant and is expensed over the award’s vesting period with a corresponding increase in additional paid-in capital. For stock options granted to non-employees, we measure the grant-date fair value based on the equity instruments issued. Compensation cost is measured when we obtain the goods, or the counterparty renders the service.
| 80 |
Financial Instruments
Credit risk
Credit risk is the risk of a loss if a customer or counterparty to a financial asset fails to meet its contractual obligations. We have credit risk relating to cash, cash equivalents and marketable securities, which we manage by dealing only with highly-rated Canadian institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents our credit exposure at the reporting date.
Currency risk
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of our business transactions denominated in currencies other than the Canadian dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in our operating results.
A portion of the expenses, mainly related to research contracts and purchase of production equipment, is incurred in U.S. dollars and in Euros, for which no financial hedging is required. There is a financial risk related to the fluctuation in the value of the U.S. dollar and the Euro in relation to the Canadian dollar. In order to minimize the financial risk related to the fluctuation in the value of the U.S. dollar in relation to the Canadian dollar, funds which were part of U.S. dollar financings continue to be invested as short-term investments in the U.S. dollar.
Furthermore, a portion of our cash and cash equivalents and marketable securities are denominated in U.S. dollars, further exposing us to fluctuations in the value of the U.S. dollar in relation to the Canadian dollar.
The following table provides an indication of our significant foreign exchange currency exposures as stated in Canadian dollars at the following dates:
| March 31, 2020 | March 31, 2019 | |||||||||||||||
| Denominated in | US $ | Euro | US $ | Euro | ||||||||||||
| Cash and cash equivalents | 5,694 | – | 3,369 | – | ||||||||||||
| Marketable securities | – | – | 2,696 | – | ||||||||||||
| Receivables | – | – | 16 | – | ||||||||||||
| Trade and other payables | (7,275 | ) | (579 | ) | (13,251 | ) | (131 | ) | ||||||||
| (1,581 | ) | (579 | ) | (7,170 | ) | (131 | ) | |||||||||
The following exchange rates are those applicable to the following periods and dates:
| March 31, 2020 | March 31, 2019 | |||||||||||||||
| Average | Reporting | Average | Reporting | |||||||||||||
| CAD$ per US$ | 1.3120 | 1.4062 | 1.3122 | 1.3349 | ||||||||||||
| CAD$ per Euro | 1.4789 | 1.5514 | 1.5192 | 1.4975 | ||||||||||||
Based on our foreign currency exposures noted above, varying the above foreign exchange rates to reflect a 5% strengthening of the U.S. dollar and Euro would have an increase (decrease) in net loss as follows, assuming that all other variables remain constant:
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Increase (decrease) in net loss | 156 | 488 | ||||||
An assumed 5% weakening of the foreign currencies would have an equal but opposite effect on the basis that all other variables remained constant.
| 81 |
Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates.
Our exposure to interest rate risk as at March 31, 2020 and March 31, 2019 is as follows:
| Cash and cash equivalents | Short-term fixed interest rate | |
| Marketable securities | Short-term fixed interest rate | |
| Unsecured convertible debentures | Short-term fixed interest rate |
Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of our short-term investments is limited because these investments have short-term maturities and are held to maturity.
Liquidity risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they fall due. We manage liquidity risk through the management of our capital structure and financial leverage. We also manage liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves our operating budgets and reviews material transactions outside the normal course of business.
Our contractual obligations related to financial instruments and other obligations and liquidity resources are presented in the liquidity and capital resources of this MD&A. See also “Note 2 - Going Concern Uncertainty” to the consolidated financial statements.
Future accounting changes
The following new standards, and amendments to standards and interpretations, are not yet effective for the period ended March 31, 2020, and have not been applied in preparing our consolidated financial statements.
In June 2016, the Financial Accounting Standards Board, or FASB, issued ASU 2016-13-Financial Instruments-Credit Losses (Topic 326), which amends guidance on reporting credit losses for assets held at amortized cost basis and available for sale debt securities. For assets held at amortized cost, the new guidance eliminates the probable initial recognition threshold in current GAAP and, instead, requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. ASU 2016-13 will affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, and any other financial assets not excluded from the scope that have the contractual right to receive cash. ASU 2016-13 is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2022. Management has not yet evaluated the impact of this ASU on the consolidated financial statements.
In August 2018, the FASB issued ASU 2018-15-Intangibles-Goodwill and Other-Internal-Use Software: Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract. ASU 2018-15 aligns the requirements for capitalizing implementation costs in such cloud computing arrangements with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. This ASU is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 and early adoption is permitted. Entities can choose to adopt the new guidance prospectively or retrospectively. Management has not yet evaluated the impact of this ASU on the consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk |
Information relating to quantitative and qualitative disclosures about market risks is detailed in “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
| 82 |
| Item 8. | Financial Statements and Supplementary Data |
See our consolidated financial statements beginning on page F-1 of this annual report on Form 10-K.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Disclosure Controls and Procedures
As of the end of the period covered by this annual report, our management, with the participation of our CEO and Vice President Finance, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15 (e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of March 31, 2020, our existing disclosure controls and procedures were effective. It should be noted that while the CEO and Vice President Finance believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Management’s Report on Internal Controls over Financial Reporting
Our management, with the participation of our CEO and Vice President Finance, is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation and fair presentation of our financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective may not prevent or detect misstatements and can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Our management conducted an assessment of the design and operation effectiveness of our internal control over financial reporting as of March 31, 2020. In making this assessment, we used the criteria established within the Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, our management has concluded that, as of March 31, 2020, our internal control over financial reporting was effective.
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the quarter ended March 31, 2020 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
We are a non-accelerated filer under the Exchange Act and not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, this annual report does not include an attestation report of our registered public accounting firm regarding our management’s assessment of internal control over financial reporting.
| Item 9B. | Other Information |
None.
| 83 |
| Item 10. | Directors, Executive Officers and Corporate Governance |
The following table sets forth information as of June 25, 2020 with respect to our directors:
| Name | Age | Position(s) held within Acasti | In Office Since | Current Term to Expire | ||||
| Directors | ||||||||
| Jan D’Alvise | 65 | President, Chief Executive Officer, Director and Corporate Secretary | June 2016 | September 2020 | ||||
| Roderick N. Carter | 56 | Chairman of the Board | October 2015 | September 2020 | ||||
| Jean-Marie (John) Canan | 63 | Director and Chairman of Audit Committee | July 2016 | September 2020 | ||||
| Donald Olds | 60 | Director and Chairman of Governance and Human Resources Committee | April 2018 | September 2020 | ||||
| Senior Management | ||||||||
| Jan D’Alvise | 65 | President, Chief Executive Officer, Director and Corporate Secretary | June 2016 | - | ||||
| Pierre Lemieux | 55 | Chief Operating Officer and Chief Scientific Officer | April 2010 | - | ||||
| Brian Groch | 53 | Chief Commercial Officer | June 2018 | - | ||||
| Jean-François Boily | 54 | Vice-President, Finance | September 2018 | - | ||||
The following is a brief biography of our current directors and senior management:
Jan D’Alvise
Ms. D’Alvise has extensive experience in the pharmaceutical, diagnostic, medical device, and drug discovery research segments of the healthcare industry. Until 2016, Ms. D’Alvise was the President and Chairman of Pediatric Bioscience, a private company that was developing a diagnostic test for Autism. Before that, she was the CEO of Gish Biomedical, a cardiopulmonary medical device company, that she sold to the Sorin Group. Prior to Gish, Ms. D’Alvise was the CEO of the Sidney Kimmel Cancer Center (SKCC), a drug discovery research institute focused on translational medicine in oncology. From 1999 until 2005, she was the Co- Founder/President/CEO/Chairman of NuGEN, Inc., and was also the Co-Founder and Executive VP/COO of Metrika Inc., from 1995 until 1999. Ms. D’Alvise built both companies from technology concept through to successful regulatory approvals, product introduction and sustainable revenue growth. Prior to 1995, Ms. D’Alvise was a VP of Drug Development at Syntex/Roche and Business Unit Director of their Pain and Inflammation business, and prior to that, VP of Commercial Operations at SYVA, (Syntex’s clinical diagnostics division). Ms. D’Alvise began her career with Diagnostic Products Corporation. Ms. D’Alvise has a B.S. in Biochemistry from Michigan Technological University. She has completed post-graduate work at the University of Michigan, Stanford University, and the Wharton Business Schools. Ms. D’Alvise has served on the board of numerous private companies and non-profits.
Dr. Roderick N. Carter
Dr. Carter has a strong history of contributions to healthcare through clinical, research, business and people leadership. He has significant experience developing and commercializing nutraceutical and pharmaceutical products and has successfully led clinical research and business development strategies for cardiovascular and inflammation related diseases. Dr. Carter is currently Principal at Aquila Life Sciences LLC, a consulting firm he founded in April 2008 focusing on pharmaceutical development and commercialization. Prior to this, he was Vice President of Clinical Development at Reliant Pharmaceuticals, which developed the OM3 cardiovascular drug LOVAZA, and today is a wholly-owned subsidiary of GlaxoSmithKline. He also served as Executive Director at Merck and Co., USA, President and Chief Executive Officer of WellGen and Senior Medical Director at Pfizer Inc., USA. Dr. Carter received his Medical Degree from the University of Witwatersrand, Johannesburg, along with a Master of Science degree in Sports Medicine from Trinity College, Dublin.
Jean-Marie (John) Canan
Mr. Canan is an accomplished business executive with over 34 years of strategic, business development and financial leadership experience. Mr. Canan recently retired from Merck & Co., Inc. where his last senior position was as Senior Vice-President, Global Controller, and Chief Accounting Officer for Merck from November 2009 to March 2014. He has managed all interactions with the audit committee of the Merck board of directors, while participating extensively with the main board and the compensation & benefits committee. Mr. Canan serves as a director of REV Group, a public company, where he chairs the audit committee and is the lead independent Director. He also serves on the board of trustees of Angkor Hospital for Children Inc. Mr. Canan is a graduate of McGill University, Montreal, Canada, and is a Canadian Chartered Accountant.
| 84 |
Donald Olds
Until May 2019, Mr. Olds was the President and Chief Executive Officer of the NEOMED Institute, an R&D organization dedicated to advancing Canadian research discoveries to commercial success. Prior to NEOMED, he was the Chief Operating Officer of Telesta Therapeutics Inc., a TSX-listed biotechnology company, where he was responsible for finance and investor relations, manufacturing operations, business development, human resources and strategy. In 2016, he led the successful sale of Telesta to a larger public biotechnology company. Prior to Telesta, he was President and Chief Executive Officer of Presagia Corp., and Chief Financial Officer and Chief Operating Officer of Aegera Therapeutics, where he was responsible for clinical operations, business development, finance, and mergers and acquisitions. At both Telesta and Aegera, Mr. Olds was responsible for raising more than $100 million in equity financing and leading regional and global licensing transactions with life sciences companies. Mr. Olds is currently Director of Goodfood Market Corp, Oxfam Quebec and Director of Presagia Corp. Since December 2019, Mr. Olds has also been the Chairman of the Board of Directors for Alfred Health Inc. He has extensive past corporate governance experience serving on the boards of private and public for-profit and not-for-profit organizations. He holds an MBA (Finance & Strategy) and M.Sc. (Renewable Resources) from McGill University.
Dr. Pierre Lemieux
Dr. Lemieux has been our Chief Operating Officer since April 12, 2010 and our Chief Scientific Officer since June 2018. Previously, Mr. Lemieux was CEO, Co-Founder and Chairman of BiolActis Inc. which he sold in 2009 to interests affiliated with the Nestlé multinational group. Mr. Lemieux joined Suprateck Pharma in 1999 as Director and Vice-President involved in the development of formulations for gene therapy on behalf of Rhone-Poulenc Rorer and Genzyme, which today are under the Sanofi banner. Prior to this, Mr. Lemieux was involved in the development of cardiovascular products at Angiotech Pharmaceuticals. Mr. Lemieux has a Ph.D. in biochemistry from Université Laval (Québec). He holds more than 16 patents and has authored over 50 publications. Mr. Lemieux’s research was conducted at Université Laval as well as at the anti-cancer center Paul Papin D’Angers (France) and the University of Nottingham (England). His research focused on ovarian cancer and its treatment with monoclonal antibodies used to target cancer drugs. After completing his graduate studies, Mr. Lemieux joined the Oncology division of the Center for Health Research, University of Texas. He obtained a postdoctoral fellowship from the Susan G. Komen Foundation (Breast Cancer). Mr. Lemieux has served on the boards of BioQuébec, Montreal in vivo and PharmaBio Development.
Mr. Brian Groch
Mr. Groch has been our Chief Commercial Officer since June 4, 2018. Mr. Groch brings over 25 years of senior experience in the healthcare and life science industries, including product commercialization, developing and executing global sales strategies, business development, and operations. Most recently, Mr. Groch served as Executive Vice President and Chief Commercial Officer at Veru Inc., a urology, oncology and female health products company, where he was responsible for leading the development and execution of the company’s long-term commercial strategy. Under his leadership, Veru experienced rapid growth in sales of the company’s women’s health product. Mr. Groch also served as Chief Commercial Officer for Telesta Therapeutics, where he led the development and implementation of the global commercial strategy. Previously, Mr. Groch served as Vice President of Commercial Operations and Market Access for Horizon Therapeutics, where he oversaw global operations including the integration of two acquisitions valued over $1.5 billion. Mr. Groch has also served as CEO and President of Exsto Therapeutics, Head of Market Access for Dendreon, and Director of Health Policy for Phadia. He has held senior management roles with Novartis and Merck & Co. He holds an M.S. in Healthcare Administration and Marketing from Central Michigan University, as well as a B.S. in Physiology from Central Michigan University.
Jean-François Boily
Mr. Boily has been our Vice-President of Finance since September 24, 2018. Prior to joining Acasti, Mr. Boily served as a Director of Finance & Information Technology at Innovaderm Research Inc., a large North American contract research organization specialized in dermatology. At Innovaderm Mr. Boily worked closely with the President and Chief Medical Officer and founder, where Mr. Boily was responsible for all aspects of Finance and IT. Mr. Boily undertook a major financial, IT and growth mandate where Mr. Boily increased revenues and profits over 25%. Prior to that, Mr. Boily was a Director of Finance at Teva Canada, a generic drug products manufacturer, where he oversaw manufacturing of generics, managing branded product launches and clinical R&D activities. At Teva, Mr. Boily worked closely with the CFO, where he had oversight of four production sites that generated more than four billion doses. Most recently, Mr. Boily worked as a consultant and Vice President of Finance and IT for a pharmaceutical start-up led by a U.S.-based investor, where he helped raise seed capital in advance of a planned initial public offering in Canada and the United States. Mr. Boily holds a BS in Accounting from HEC Montreal and is a Chartered Public Accountant.
| 85 |
Family Relationships
There are no family relationships between any directors or officers of the Company.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires directors, executive officers, and shareholders owning more than 10% of any class of a company’s outstanding equity shares to file reports of ownership and changes of ownership with the SEC. As of April 1, 2020, we are required to comply with Section 16(a) because we are no longer eligible to rely upon foreign private issuer exemptions under U.S. securities laws and NASDAQ’s corporate governance rules.
Based solely upon its review of the copies of such forms it received, or written representations from certain reporting persons for whom no such forms were required, we are aware of no late Section 16(a) filings.
Code of Business Conduct and Ethics
Please see the section entitled “Code of Business Conduct and Ethics” in “Item 13. Certain Relationships and Related Transactions and Director Independence.”
Audit Committee
Our audit committee is responsible for assisting the board of directors in fulfilling its oversight responsibilities with respect to financial reporting, including:
| · | reviewing our procedures on overall financial reporting and internal control framework. |
| · | reviewing and approving the engagement of the auditor. |
| · | reviewing annual and quarterly financial statements and all other material continuous disclosure documents, including our annual information form and management’s discussion and analysis. |
| · | assessing our financial and accounting personnel. |
| · | assessing our accounting policies. |
| · | reviewing our risk management procedures; and |
| · | reviewing any significant transactions outside our ordinary course of business and any pending litigation involving us. |
The audit committee has direct communication channels with our management performing financial functions and our external auditor, to discuss and review such issues as the audit committee may deem appropriate. As of March 31, 2020, the audit committee was composed of Mr. Canan, as chairperson, Dr. Carter and Mr. Olds. Each of Mr. Canan, Dr. Carter and Mr. Olds is “financially literate” and “independent” within the meaning of the Exchange Act. As of the date of this annual report, the composition of the audit committee remains the same as at March 31, 2020.
Audit Committee Financial Expert
Our board of directors has determined that Mr. Canan is the “audit committee financial expert”, as defined by applicable regulations of the SEC. The SEC has indicated that the designation of Mr. Canan as an audit committee financial expert does not make him an “expert” for any purpose, impose any duties, obligations or liability on Mr. Canan that are greater than those imposed on members of the audit committee and board of directors who do not carry this designation or affect the duties, obligations or liability of any other member of the audit committee or board of directors.
| 86 |
| Item 11. | Executive Compensation |
Summary of our Compensation Programs
Our executive compensation program is intended to attract, motivate and retain high-performing senior executives, encourage and reward superior performance, and align the executives’ interests with ours as well as shareholders by providing compensation that is competitive with the compensation received by executives employed by comparable companies, and ensuring that the achievement of annual objectives is rewarded through the payment of bonuses, and providing executives with long-term incentive through the grant of stock options.
Our governance and human resources committee, or GHR committee, has authority to retain the services of independent compensation consultants to advise its members on executive and board compensation and related matters, and to determine the fees and the terms and conditions of the engagement of those consultants. During our fiscal year ended March 31, 2020, the GHR committee retained compensation consulting services from FW Cook to review our executive compensation programs, including base salary, short-term and long-term incentives, total cash compensation levels and total direct compensation of certain senior positions, against those of peer groups of similar and larger size, as measured by market capitalization, biotechnology and pharmaceutical companies listed or headquartered in North America. The consultants also reviewed board compensation, including advisory fees and equity incentives. All of the services provided by the consultants were provided to the GHR committee. The GHR committee assessed the independence of the consultants and concluded that its engagement of the consultants did not raise any conflict of interest with us or any of our directors or executive officers.
Compensation for all named executive officers was below the peer company median following FW Cook’s review during fiscal period 2020.
Use of Fixed and Variable Pay Components
Compensation of our named executive officers, or NEOs, is revised each year and has been structured to encourage and reward executive officers on the basis of short-term and long-term corporate performance. In the context of its analysis of compensation for our fiscal year ended March 31, 2020, the following components were examined by the GHR committee:
| · | base salary; |
| · | short term incentive plan, consisting of a cash bonus; |
| · | long term incentive plan, consisting of stock options and equity incentive grants based on performance and/or time vesting conditions; and |
| · | other elements of compensation, consisting of group benefits and perquisites. |
Base Salary
We intend to be competitive over time, with comparator companies and to attract and retain top talent. The GHR committee reviews compensation periodically to be sure that it meets this strategic imperative. Base salary is set to reflect an individual’s skills, experience and contributions within a salary structure consistent with peer group data, and with our gender pay equity policy. Base salary structure is revised annually by the GHR committee as our financial and market conditions evolve.
Short Term Incentive Plan (STIP)
Our Short-Term Incentive Plan, or STIP, provides for potential rewards when a threshold of corporate performance is met. Personal objectives that support corporate goals are established annually with each employee and are assessed at the end of each financial year. Personal objectives are assessed through a performance grid, with pre-specified, objective performance criteria. STIP awards are paid out in proportion to overall company performance which establishes the STIP pool, and individual performance, which is determined in end-of-year performance reviews. For the most senior participants in the STIP, greater weight is assigned to corporate objectives. Target payout is expressed as a percentage of base salary, and is determined by benchmarking against peer group data, and board discretion. Annual salary for STIP purposes is the annual salary in effect at the end of the plan year (i.e., prior to any annual salary increases awarded for the subsequent year).
| 87 |
The STIP is a discretionary variable compensation plan, and all STIP payments are subject to board approval. Participants must be employed by us at the end of the financial year to qualify. We reserve the right to modify or discontinue the STIP at any time.
Ms. D’Alvise, our CEO, is eligible for up to a 50% bonus of her annual base salary. Dr. Lemieux, our COO, and Mr. Groch, our CCO, are each eligible for up to a 40% bonus of their annual base salary. Mr. Boily, our Vice-President, Finance, is eligible for up to a 30% bonus of his annual base salary.
These performance goals will take into account the achievement of corporate milestones within timelines and budget and individual objectives determined annually by the board according to short-term priorities.
Long Term Incentive Plan (LITP)
The LTIP has been adopted as a reward and retention mechanism. Participation is determined annually at the discretion of the board. Employees approved by our board of directors may participate in our stock option plan, which is designed to align the long-term interests of participants with those of shareholders, in order to promote shareholder value. The GHR committee may also determine, in its sole discretion, ad hoc stock option awards to be granted to participants in order to address extraordinary situations. Awards at any level may be adjusted as necessary to maintain an equity burn rate and overhang similar to comparator companies. In addition to our stock option plan, the board is also empowered to grant ad hoc awards, from time to time, under our equity incentive plan to provide for a share-related mechanism to attract, retain and motivate qualified directors, senior employees and consultants.
The GHR committee determines the number of stock options to be granted to a participant based on peer group data and taking into account corporate performance and the employee’s level in the organization. The LTIP calculation for NEOs is determined from both reviewing grant values and a dilution-based methodology that considers the annual grant rate as a percent of shares outstanding.
Grant values during fiscal period 2020 were below the peer group median, although the grant rate as a percent of shares outstanding was near the peer median. Awards are subject to adjustment by the board in reviewing annual achievement of corporate performance and availability of shares.
Our directors and executive officers are not permitted to purchase financial instruments, such as prepaid variable forward contracts, equity swaps, collars or units of exchange funds that are designed to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by the director or officer.
Share Ownership Guidelines
To further align the interests of our executives and board members with those of our other shareholders, the board has adopted share ownership guidelines. Under these guidelines, non-employee directors, the CEO and other executives (i.e., CFO, COO, VPs) are required to retain and hold 50% of the shares acquired by them under any equity incentive award granted on or after June 7, 2017 (after subtracting shares sold to pay for option exercise costs, and relevant federal, state, and local taxes which are assumed to be at the highest marginal tax rates). In addition, the share retention rule applies unless the executive or non-employee director beneficially owns shares with a value at or in excess of the following share ownership guidelines:
| · | Non-employee directors — 2x then-current total annual cash retainer |
| · | CEO — 2x then-current annual base salary |
| · | Other executives — 1x then-current annual base salary. |
The value of an individual’s shares for purposes of the share ownership guidelines is deemed to be the greater of the then- current fair market value of the shares, or the individual’s cost basis in the shares. Shares counted in calculating the share ownership guidelines include shares beneficially owned outright, whether from open market purchases, shares retained after option exercises, and shares of restricted stock or deferred stock units that have fully vested. In addition, in the case of vested, unexercised, in-the-money stock options, the in-the-money value of the stock options will be included in the share ownership calculation. Executives have five years from their date of hire or promotion to satisfy the share ownership guidelines.
| 88 |
Stock Option Plan
Our stock option plan was adopted by our board of directors on October 8, 2008 and has been amended from time to time, as most recently amended on April 15, 2019 and approved by shareholders on August 27, 2019. The grant of options is part of the long-term incentive component of executive and director compensation and an essential part of compensation. Qualified directors, employees and consultants may participate in our stock option plan, which is designed to encourage option holders to link their interests with those of our shareholders, in order to promote an increase in shareholder value. Awards and the determination of any exercise price are made by our board of directors, after recommendation by the GHR committee. Awards are established, among other things, according to the role and responsibilities associated with the participant’s position and his or her influence over appreciation in shareholder value. Any award grants a participant the right to purchase a certain number of common shares during a specified term in the future, after a vesting period and/or specific performance conditions, at an exercise price equal to at least 100% of the market price (as defined below) of our common shares on the grant date. The “market price” of common shares as of a particular date generally means the highest closing price per common share on the TSXV, NASDAQ, or any other exchange on which the common shares are listed from time to time, for the last preceding date on which there was a sale of common shares on that exchange (subject to certain exceptions set forth in the stock option plan in the event that we are no longer traded on any stock exchange). Previous awards may sometimes be taken into account when new awards are considered.
In accordance with the stock option plan, all of an option holder’s options will immediately fully vest on the date of a Change of Control event (as defined in the stock option plan), subject to the terms of any employment agreement or other contractual arrangement between the option holder and us.
However, in no case will the grant of options under the plan, together with any proposed or previously existing security based compensation arrangement, result in (in each case, as determined on the grant date): the grant to any one consultant within any 12-month period, of options reserving for issuance a number of common shares exceeding in the aggregate 2% of our issued and outstanding common shares (on a non-diluted basis); or the grant to any one employee, director and/or consultant, which provides investor relations services, within any 12-month period, of options reserving for issuance a number of common shares exceeding in the aggregate 2% of our issued and outstanding common shares (on a non-diluted basis).
Options granted under the stock option plan are non-transferable and are subject to a minimum vesting period of 36 months for management, and 18 months for non-executive board members, in each case with gradual and equal vesting on no less than a quarterly basis. They are exercisable, subject to vesting and/or performance conditions, at a price equal to the highest closing price of the common shares on the TSXV, NASDAQ, or any other exchange on which the common shares are listed from time to time, on the day prior to the grant of such options. In addition, and unless otherwise provided for in the agreement between us and the holder, options will also lapse upon termination of employment or the end of the business relationship with us except that they may be exercised for 60 days after termination, ceasing to hold office or the end of the business relationship (30 days for investor relations services employees), in each case to the extent that they will have vested on such date of termination of employment, end of the business relationship or ceasing to hold office, as applicable, except in the case of death, disability or retirement where this period is extended to 12 months.
Subject to the approval of relevant regulatory authorities, including the TSXV, NASDAQ, if applicable, and compliance with any conditions attached to that approval (including, in certain circumstances, approval by disinterested shareholders) if applicable, the board of directors has the right to amend or terminate the stock option plan. However, unless option holders consent to the amendment or termination of the stock option plan in writing, any such amendment or termination of the stock option plan cannot affect the conditions of options that have already been granted and that have not been exercised under the stock option plan.
Options for common shares representing a fixed rate of 15% of our outstanding issued common shares as of April 9, 2019 may be granted by the board under the stock option plan. As of the date of this annual report, there were 11,719,910 common shares reserved for issuance under the stock option plan and 9,936,486 options outstanding under the stock option plan.
| 89 |
Equity Incentive Plan
On May 22, 2013, our equity incentive plan was adopted by the board in order to, among other things, provide us with a share-related mechanism to attract, retain and motivate qualified directors, employees and consultants. The adoption of the equity incentive plan was initially approved by shareholders at our 2013 Shareholders’ meeting held on June 27, 2013 and has been amended from time to time, as most recently amended on August 26, 2019 and approved by shareholders on August 27, 2019.
Eligible persons may participate in the equity incentive plan. “Eligible persons” under the equity incentive plan consist of any director, officer, employee or consultant (as defined in the equity incentive plan) of our Company or a subsidiary who may participate in the equity incentive plan. A participant is an eligible person to whom an award has been granted under the equity incentive plan. The equity incentive plan provides us with the option to grant to eligible persons bonus shares, restricted shares, restricted share units, performance share units, deferred share units and other share-based awards.
If, and for so long as our common shares are listed on the TSXV, no more than 2% of the issued and outstanding common shares may be granted to any one consultant or employee conducting investor relations activities in any 12-month period.
The board has the right to determine that any unvested or unearned restricted share units, deferred share units, performance share units or other share-based awards or restricted shares subject to a restricted period outstanding immediately prior to the occurrence of a change in control will become fully vested or earned or free of restriction upon the occurrence of a change in control. The board may also determine that any vested or earned restricted share units, deferred share units, performance share units or other share-based awards will be cashed out at the market price as of the date a change in control is deemed to have occurred, or as of such other date as the board may determine prior to the change in control. Further, the board has the right to provide for the conversion or exchange of any restricted share unit, deferred share unit, performance share unit or other share-based award into or for rights or other securities in any entity participating in or resulting from the change in control.
The equity incentive plan is administered by the board and the board has sole and complete authority, in its discretion, to determine the type of awards under the equity incentive plan relating to the issuance of common shares (including any combination of bonus shares, restricted share units, performance share units, deferred share units, restricted shares or other share-based awards) in such amounts, to such persons and under such terms and conditions as the board may determine, in accordance with the provisions of the equity incentive plan and the recommendations made by the GHR committee.
Subject to the adjustment provisions provided for in the equity incentive plan and the applicable rules and regulations of all regulatory authorities to which we are subject (including any stock exchange), the total number of common shares reserved for issuance pursuant to awards granted under the equity incentive plan will be equal to a number that (A) if, and for so long as the common shares are listed on the TSXV, will not exceed the lower of (i) 1,953,318 common shares, and (ii) 15% of the issued and outstanding common shares, which as of April 9, 2019, representing 11,719,910 common shares, which includes common shares issuable pursuant to options issued under our stock option plan.
Other Forms of Compensation
Retirement Plans. Effective June 1, 2016, we sponsor a voluntary Registered Retirement Savings Plan, or RRSP, matching program, which is open to all eligible employees, including NEOs who reside in Canada. The RRSP matching program matches employees’ contributions up to a maximum of $1,500 per fiscal year for eligible employees who participate in the program. Effective January 1, 2019, a 401K plan was implemented for US employees. Because of the small size of our current employee population in the US and to assure passage of anti-discrimination testing, the 401K administrator, TransAmerica, required either a 4% match or a 3% “safe harbor” contribution. Balancing cost considerations with a plan design that is both externally competitive and internally equitable, Acasti adopted the “safe harbor” provision which provides a contribution of 3% of salary to the 401K accounts of all eligible US employees, including NEOs who reside in the US.
| 90 |
Other Benefits and Perquisites. Our executive employee benefit program also includes life, medical, dental and disability insurance. These benefits and perquisites are designed to be competitive overall with equivalent positions in comparable organizations. We do not have a pension plan for employees.
Compensation Governance
Compensation of our executive officers and directors is recommended to the board of directors by the GHR committee. In its review process, the GHR committee informally reviews executive and corporate performance on a quarterly basis, with input from management. Annually, the GHR committee conducts a more formal review and assessment of executive and corporate performance. During the fiscal year ended March 31, 2020, the GHR committee was composed of the following members, each of whom is independent: Mr. Olds (Chairman), Dr. Carter, and Mr. Canan. The GHR committee establishes management compensation policies, and oversees their general implementation. All members of the GHR committee have direct experience, which is relevant to their responsibilities as GHR committee members. All members are or have held senior executive or director roles within significant businesses in our industry, several also having public companies experience, and have a good financial understanding which allows them to assess the costs versus benefits of compensation plans. The GHR committee’s members combined experience in our sector provides them with a good understanding of our success factors and risks, which is very important when determining metrics for measuring success.
Risk management is a primary consideration of the GHR committee when implementing its compensation program. We do not believe that our compensation program results in unnecessary or inappropriate risk taking, including risks that are likely to have a material adverse effect on us. Payments of bonuses, if any, are not made unless performance goals are met.
For executives, more than half of their target compensation (base salary + target STIP awards + target LTIP awards) is considered “at risk”. We believe this mix results in a strong pay-for-performance relationship and alignment with shareholders, and is competitive with other firms of comparable size in similar fields. The CEO (or any person acting in that capacity) makes recommendations to the GHR committee as to the compensation of our executive officers, other than herself for review and approval by the board. The GHR committee makes recommendations to the board of directors as to the compensation of the CEO, for approval. The CEO’s salary is based on comparable market consideration, and the GHR committee’s assessment of her performance, with regard to our financial performance, and progress in achieving key strategic business goals.
Qualitative factors beyond the quantitative financial metrics are also a key consideration in determination of individual executive compensation payments. How executives achieve their financial results and demonstrate leadership consistent with our values are key to individual compensation decisions.
| 91 |
Compensation Paid to Named Executive Officers
The following table sets forth the compensation information for our principal executive officers, and our most highly paid executive officers, during the fiscal years ended March 31, 2020, and 2019, respectively.
| Name and | Year | Salary | Bonus ($) | Stock | Option | Nonequity | All Other | Total |
| Principal | ($) | Awards | Awards | Incentive | Compensation | Compensation | ||
| Position | ($) | ($)(1) (2) | Plans | ($) | ($) | |||
| ($) | ||||||||
| Jan D’Alvise | March 31, 2020 | 410,703 | 154,781 | - | 1,620,863 | - | - | 2,186,347 |
| President and CEO | March 31, 2019 | 372,919 | 180,566 | - | 372,070 | - | - | 925,555 |
| Pierre Lemieux | March 31, 2020 | 264,128 | 80,018 | - | 603,458 | - | - | 947,604 |
| COO | March 31, 2019 | 195,329 | 74,308 | - | 150,066 | - | - | 419,703 |
| Brian Groch | March 31, 2020 | 289,615 | 87,000 | - | 357,461 | - | - | 734,106 |
| CCO | March 31, 2019 | 221,268 | 66,500 | - | 123,168 | - | - | 410,936 |
______________________________
Notes:
(1) The fair value of stock options is estimated at the grant date using the Black-Scholes option pricing model. This model requires the input of a number of parameters, including share price, share exercise price, expected share price volatility, expected time until exercise and risk-free interest rates. Although the assumptions used reflect management’s best estimates, they involve inherent uncertainties based on market conditions generally outside of our control
(2) The fair value of the option-based awards granted on July 2, 2018 was CAD$0.54. The fair value of the stock-based awards granted on April 15, 2019 was CAD$ 0.91; the fair value of the stock-based granted on August 27, 2019 was CAD$1.85 and the fair value of the stock-based awards granted on March 31, 2020 was CAD$0.41.
Outstanding Equity Awards at March 31, 2020
The following tables provide information about the number and value of the outstanding option-based awards held by the NEOs as of March 31, 2020.
| Option awards | ||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) (1) | Option expiration date | |||||||||||||
| 525,000 | – | – | $ | 1.56 | May 12, 2023 | |||||||||||||
| 172,000 | 86,000 | 86,000 | $ | 1.77 | June 14, 2027 | |||||||||||||
| Jan D’Alvise | 114,667 | 57,333 | 57,333 | $ | 1.77 | June 14, 2027 | ||||||||||||
| 453,124 | 453,124 | 453,124 | $ | 0.77 | July 2, 2028 | |||||||||||||
| 56,525 | 169,575 | 169,575 | $ | 1.28 | April 15, 2029 | |||||||||||||
| 192,975 | 578,975 | 578,975 | $ | 1.28 | April 15, 2029 | |||||||||||||
| 1,335,000 | 1,335,000 | $ | 0.53 | March 31, 2030 | ||||||||||||||
| 16,900 | – | – | $ | 4.50 | June 1, 2022 | |||||||||||||
| 31,400 | – | – | $ | 1.99 | May 30, 2023 | |||||||||||||
| 50,000 | – | – | $ | 1.65 | February 24, 2027 | |||||||||||||
| Pierre Lemieux | 62,000 | 31,000 | 31,000 | $ | 1.77 | June 14, 2027 | ||||||||||||
| 41,333 | 20,667 | 20,667 | $ | 1.77 | June 14, 2027 | |||||||||||||
| 182,757 | 182,757 | 182,757 | $ | 0.77 | July 2, 2028 | |||||||||||||
| 19,825 | 59,475 | 59,475 | $ | 1.28 | April 15, 2029 | |||||||||||||
| 67,675 | 203,025 | 203,025 | $ | 1.28 | April 15, 2029 | |||||||||||||
| – | 587,000 | 587,000 | $ | 0.53 | March 31, 2030 | |||||||||||||
| 150,000 | 150,000 | 150,000 | $ | 0.77 | July 2, 2028 | |||||||||||||
| Brian Groch | 8,500 | 25,500 | 25,500 | $ | 1.28 | April 15, 2029 | ||||||||||||
| 29,000 | 87,000 | 87,000 | $ | 1.28 | April 15, 2029 | |||||||||||||
| – | 587,000 | 587,000 | $ | 0.53 | March 31, 2030 | |||||||||||||
______________________________
Notes:
(1) Canadian dollars.
| 92 |
Employment Agreements with Named Executive Officers
Jan D’Alvise, President and CEO
On May 11, 2015, we entered into an executive employment agreement with Ms. D’Alvise. Pursuant to her executive employment agreement, Ms. D’Alvise’s annual base salary was set at $330,000 and she is eligible to receive annual performance bonuses based on target amount of 40% of her annual base salary with a maximum of up to 80% of her annual base salary. In accordance with the terms and provisions of the executive employment agreement we entered into with Ms. D’Alvise, we may terminate the executive’s employment at any time for “good and sufficient cause”, as defined in the employment agreement, without notice or severance. We may terminate the executive’s employment at any time without cause or upon a change of control, as defined in our Stock Option Plan, by providing the executive with sixty days’ notice of termination and payment equal to twelve months’ base salary plus any bonus payable. The executive may decide to resign from employment and must provide us with at least sixty days' advance written notice. The executive may decide to terminate employment with “good reason”, as defined in the employment agreement, and we are required to make payment equal to twelvemonths’ base salary plus any bonus payable.
Pierre Lemieux, COO
On September 26, 2017, we entered into an executive employment agreement with Dr. Lemieux. Pursuant to his executive employment agreement, Dr. Lemieux’s annual base salary was set at CDN$253,700 and he is eligible to receive annual performance bonuses of up to 40% of his annual base salary. In accordance with the terms and provisions of the executive employment agreement we entered into with Dr. Lemieux, we may terminate the executive’s employment at any time for “good and sufficient cause”, as defined in the employment agreement, without notice or severance. We may terminate the executive’s employment at any time without cause or upon a change of control, as defined in our Stock Option Plan, by providing the executive with thirty days’ notice of termination and payment equal to twelve months’ base salary plus any bonus payable. The executive may decide to resign from employment and must provide us with at least sixty days' advance written notice. The executive may decide to terminate employment with “good reason”, as defined in the employment agreement, and we are required to make payment equal to twelve months of base salary.
Brian Groch, CCO
On May 31, 2018, we entered into an executive employment agreement with Mr. Groch. Pursuant to his executive employment agreement, Mr. Groch’s annual base salary was set at $280,000 and he is eligible to receive annual performance bonuses of up to 40% of his annual base salary. In accordance with the terms and provisions of the executive employment agreement we entered with Mr. Groch, we may terminate the executive’s employment at any time for “good and sufficient cause”, as defined in the employment agreement, without notice or severance. We may terminate the executive’s employment at any time, for any reason, with or without notice. Similarly, the employee has the right to terminate his employment with us at any time for any reason, with or without notice. The employee also has the right to terminate his employment with us upon the occurrence of “constructive termination” as defined in the employment agreement. Should we terminate the employee’s employment without cause or should the employee terminate his employment as a result of constructive termination, we will pay the employee an amount equal to six months of base salary. Should the employee’s employment be terminated without cause upon a change of control event, as defined in our Stock Option Plan, we will pay the employee an amount equal to twelve months of base salary.
| 93 |
Compensation of Directors
Our directors’ compensation consists of an annual fixed compensation of $60,000 for the chairman of the board and $30,000 for the other non-executive board members. In addition, the chairperson of the audit committee and the chairperson of the governance and human resources committee receive additional compensation of $15,000 and $10,000, respectively, while members of the audit committee and the governance and human resources committee receive additional compensation of $7,500 and $5,000, respectively. The directors are also entitled to a fee of $1,000 per non-regularly scheduled board meeting as well as a reimbursement for travelling and other reasonable expenses properly incurred by them in attending meetings of the board or any committee or in otherwise serving us, in accordance with our policy on travel and expenses.
Following their first election to our board of directors, non-executive directors are eligible to receive an initial equity grant of up to 150% of their annual cash retainer worth of stock options vesting monthly in equal installments over a 12-month period, subject to the other terms and conditions set forth under the heading “Stock Option Plan”. In addition to their initial grant, non-executive directors are eligible to receive an annual equity-based award equal to 100% of their total annual cash retainer vesting monthly in equal installments over a 12-month period. These awards will be granted at the same time that we are performing our annual performance review for our employees, subject to availability of common shares and subject to the terms and conditions described under the headings “Stock Purchase Plan” and “Equity Incentive Plan”. The level of these awards will be consistent with equivalent awards in comparable companies obtained from the benchmark exercise and in accordance with the recommendations obtained from our independent compensation consultant.
The total compensation for our non-executive directors during fiscal year ended March 31, 2020 was as follows:
| Name | Fees earned or | Stock | Option | Non-equity incentive plan | Nonqualified deferred compensation | All other | ||||||||||||||||||||||
| paid in cash | awards | awards | compensation | earnings | compensation | Total | ||||||||||||||||||||||
| ($) | ($) | ($)(1) | ($) | ($) | ($) | ($) | ||||||||||||||||||||||
| Roderick N. Carter(5) | 72,500 | – | 12,059 | (2) | – | – | – | 194,032 | ||||||||||||||||||||
| 84,123 | (3) | |||||||||||||||||||||||||||
| 25,351 | (4) | |||||||||||||||||||||||||||
| Jean-Marie (John) Canan(6) | 50,000 | – | 9,061 | (2) | – | – | – | 147,539 | ||||||||||||||||||||
| 63,127 | (3) | |||||||||||||||||||||||||||
| 25,351 | (4) | |||||||||||||||||||||||||||
| Donald Olds(7) | 47,500 | – | 8,039 | (2) | – | – | – | 137,065 | ||||||||||||||||||||
| 56,175 | (3) | |||||||||||||||||||||||||||
| 25,351 | (4) | |||||||||||||||||||||||||||
_____________________________
Notes:
(1) The fair value of the awards is estimated at the grant date using the Black-Scholes option pricing model. This model requires the input of a number of parameters, including share price, share exercise price, expected share price volatility, expected time until exercise and risk-free interest rates. Although the assumptions used reflect management’s best estimates, they involve inherent uncertainties based on market conditions generally outside of our control.
(2) Represents share options granted on April 15, 2019 under the Stock Option Plan with an exercise price of C$1.28. These share options vest in 6 equal installments on a quarterly basis starting from April 15, 2019 until October 15, 2020.
(3) Represents share options grant on April 15, 2019 under the Stock Option Plan with an exercise price of C$1.28 and approved by the AGM held on August 27, 2019. These share options vest in 18 equal installments on a monthly basis starting from April 15, 2019 until October 15, 2020.
(4) Represents share options grant on March 31, 2020 under the Stock Option Plan with an exercise price of C$0.53. These share options vest in 18 equal installments on a monthly basis starting from March 31, 2020 until March 31, 2021.
(5) Dr. Carter earned a director compensation of $72,500.
(6) Mr. Canan earned a director compensation of $50,000.
(7) Mr. Olds earned a director compensation of $47,500.
| 94 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
Equity Compensation Plan Information
The following table sets forth certain information regarding the Company’s equity compensation plans as of March 31, 2020:
| Plan category | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights | (b) Weighted-average exercise price of outstanding options, warrants and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders (Stock Option Plan)(1): | 9,936,486 | CAD$1.00 | 1,329,382 | |||||||||
| Equity compensation plans approved by security holders (Equity Incentive Plan)(2): | – | $– | – | |||||||||
| Equity compensation plans not approved by security holders (Stock Option Plan): | – | $– | – | |||||||||
| Equity compensation plans not approved by security holders (Equity Incentive Plan): | – | $– | – | |||||||||
| Total | 9,936,486 | CAD$1.00 | 1,329,382 |
______________________________
Notes:
(1) A summary of certain material provisions of the Company’s Stock Option Plan is available under “Item 11. Executive Compensation – Summary of our Compensation Programs – Stock Option Plan”.
(2) The total number of common shares reserved for issuance under the Company’s Equity Incentive Plan is limited by the number of options that are outstanding under the Stock Option Plan such that the total number of common shares available for issuance under both stock-based compensation plans shall not exceed 11,719,910. A summary of certain material provisions of the Company’s Equity Incentive Plan is available under “Item 11. Executive Compensation – Summary of our Compensation Programs – Equity Incentive Plan”.
Security ownership of certain beneficial owners
The following table sets forth certain information regarding beneficial ownership of our common shares as of May 31, 2020 by each director and the executive officer identified above, and all directors and executive officers as a group. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. All common shares are common shares with the same voting rights.
| 95 |
For the purposes of calculating percent ownership, as of May 31, 2020, 90,209,449 common shares were issued and outstanding, and, for any individual who beneficially owns shares represented by options exercisable within sixty days of May 31, 2020, these shares are treated as if outstanding for that person, but not for any other person.
| Name and Address of Beneficial Owner (1) | Amount and Nature of Beneficial Ownership | Percentage of Common Shares | ||||||
| Jan D’Alvise | 1,725,479 (2) | 1.9% | ||||||
| Roderick N. Carter | 376,510 (3) | * | ||||||
| Jean-Marie (John) Canan | 268,750 (4) | * | ||||||
| Donald Olds | 122,800 (5) | * | ||||||
| Brian J. Groch | 225,000 (6) | * | ||||||
| Pierre Lemieux | 538,516 (7) | * | ||||||
| Jean-François Boily | 135,600 (8) | * | ||||||
| Directors and officers as a group (7 persons) | 3,392,655 | 3.8% | ||||||
____________
* Less than 1%.
Notes:
(1) Unless otherwise indicated, the address of each of the executive officers and directors named above is 545 Promenade du Centropolis, Suite 100, Laval Québec, Canada H7T 0A3.
(2) Includes 1,672,979 common shares that Jan D’Alvise may acquire through the exercise of share options within 60 days hereof.
(3) Includes 376,510 common shares that Roderick N. Carter may acquire through the exercise of share options within 60 days hereof.
(4) Includes 168,750 common shares that Jean-Marie (John) Canan may acquire through the exercise of share options within 60 days hereof.
(5) Includes 84,800 common shares that Donald Olds may acquire through the exercise of share options within 60 days hereof. Includes 38,000 common shares held and controlled by Mr. Olds’ spouse, Ofra Aslan.
(6) Includes 225,000 common shares that Brian Groch may acquire through the exercise of share options within 60 days hereof.
(7) Includes 531,516 common shares that Pierre Lemieux may acquire through the exercise of share options within 60 days hereof.
(8) Includes 125,000 common shares that Jean-François Boily may acquire through the exercise of share options within 60 days hereof.
To the best of our knowledge, there are no beneficial owners of 5% or more of any class of our voting securities, other than Acuitas Group Holdings, LLC (which is controlled by Terren S. Peizer), located at 2120 Colorado Avenue, #230, Santa Monica, California 90404, which, according to a beneficial ownership report on Schedule 13G filed with the SEC on July 19, 2019, beneficially owns (i) 5,000,000 of our common shares and (ii) 3,650,000 of our common shares issuable upon the exercise of currently exercisable warrants.
Changes in Control
There existed no change in control arrangements at March 31, 2020.
| Item 13. | Certain Relationships and Related Transactions and Director Independence |
Related Transactions
None.
Director Independence
Our board of directors believes that, in order to maximize its effectiveness, the board must be able to operate independently. A majority of directors must satisfy the applicable tests of independence, such that the board of directors complies with all independence requirements under applicable corporate and securities laws and stock exchange requirements applicable to us. No director will be independent unless the board of directors has affirmatively determined that the director has no material relationship with us or any of our affiliates, either directly or indirectly or as a partner, shareholder or officer of an organization that has a relationship with us or our affiliates. Such determinations will be made on an annual basis and, if a director joins the board of directors between annual meetings, at such time.
Independent Directors
The board of directors determined that Mr. Canan, Dr. Carter and Mr. Olds are independent within the meaning of NI 52-110 and NASDAQ Stock Market rules.
Directors Who are Not Independent
The board of directors determined that Ms. D’Alvise is not independent within the meaning of NI 52-110 and NASDAQ Stock Market rules given that she is our President and Chief Executive Officer.
| 96 |
During the fiscal year ended March 31, 2020, the board of directors held 16 meetings. All directors were in attendance for each regularly scheduled quarterly and annual meeting of the Board.
Chairman of the Board
Dr. Carter acts as chairman of the board. His duties and responsibilities consist of the oversight of the quality and integrity of the board of directors’ practices.
Board Mandate
The board of directors is responsible for overseeing management in carrying out the business and affairs of the Company. Directors are required to act and exercise their powers with reasonable prudence in the best interests of the Company. The board agrees with and confirms its responsibility for overseeing management's performance in the following particular areas:
| • | approving and monitoring the Company’s compliance procedures; |
| • | establishing and developing of the Company’s corporate governance principles and committees; |
| • | evaluating the strategic plan of the Company; |
| • | identification and oversight of the principal risks associated with the business of the Company and application of appropriate systems to manage and mitigate such risks; |
| • | planning for succession of management; |
| • | the Company's policies regarding communications with its shareholders and others; and |
| • | the integrity of the internal controls and management information systems of the Company. |
In carrying out its mandate, the board relies primarily on management to provide it with regular detailed reports on the operations of the Company and its financial position. The board reviews and assesses these reports and other information provided to it at meetings of the board and/or of its committees. At least annually, the board approves a strategic plan for the Company taking into account, among other things, the opportunities and risks of the Company’s business, its risk appetite, emerging trends, and the competitive environment in the industry.
Position Descriptions
Written position description have been approved for the chairs of each committee of the board of directors. The primary role and responsibility of the chair of each committee of the board of directors is to: (i) in general, ensure that the committee fulfills its mandate, as determined by the board of directors and in accordance with the committee’s charter; (ii) chair meetings of the committee; (iii) report to the board of directors; and (iv) act as liaison between the committee and the board of directors and our management.
The board of directors has adopted a written position description for the chairman of the board of directors.
Chairman of the Board
The chairman of the board of directors is responsible for leading the board to fulfill its duties under the board’s mandate as independent of management, and acting as an advisor to the chief executive officer.
The chairman’s duties include, but are not limited to, setting meeting agendas, approving and supervising management’s progress towards achieving strategic goals, chairing meetings and working with the respective committee and management to ensure, to the greatest extent possible, the effective functioning of the committee and the board of directors. The chairman must oversee that the relationship between the board of directors, management of the Company, the Company’s shareholders and other stakeholders are effective, efficient and further to the best interests of the Company.
Orientation and Continuing Education
We provide orientation for new appointees to the board of directors and committees in the form of informal meetings with members of the board and senior management, complemented by presentations on the main areas of our business. The board does not formally provide continuing education to its directors, as directors are experienced members. The board of directors relies on third party professional assistance, when judged necessary, in order to be educated/updated on a particular topic.
| 97 |
Code of Business Conduct and Ethics
The board of directors adopted a Code of Business Conduct and Ethics, or Code of Conduct, for our directors, officers and employees on May 31, 2007, as amended from time to time. Our Code of Conduct can be found on SEDAR at www.sedar.com and on our web site on www.acastipharma.com. A copy of the Code of Conduct can also be obtained by contacting our corporate secretary. Since its adoption by the board of directors, any breach of the Code of Conduct must be brought to the attention of the board of directors by our CEO or other senior executives. No report has ever been filed which pertains to any conduct of a director or executive officer that constitutes a breach to our Code of Conduct.
Since the adoption of the Code of Conduct and the following policies, the board of directors actively monitors compliance with the Code Conduct and promotes a business environment where employees are encouraged to report malfeasance, irregularities and other concerns. The Code of Conduct provides for specific procedures for reporting non-compliant practices in a manner which, in the opinion of the board of directors, encourages and promotes a culture of ethical business conduct.
The board of directors also adopted a disclosure policy, insider trading policy, majority voting policy, management and board compensation policies, and a whistleblower policy.
In addition, under the Civil Code of Québec, to which we are subject as a legal person incorporated under the Business Corporations Act (Québec) (L.R.Q., c. S-31), a director must immediately disclose to the board any situation that may place him or her in a conflict of interest. Any such declaration of interest is recorded in the minutes of proceeding of the board of directors. The director abstains, except if required, from the discussion and voting on the question. In addition, it is our policy that an interested director recuse himself or herself from the decision-making process pertaining to a contract or transaction in which he or she has an interest.
Nomination of Directors
The board of directors receives recommendations from the GHR committee, but retains responsibility for managing its own affairs by, among other things, giving its approval for the composition and size of the board of directors, and the selection of candidates nominated for election to the board of directors. The GHR committee initially evaluates candidates for nomination for election as directors, having regard to the background, employment and qualifications of possible candidates.
The selection of the nominees for the board of directors is made by the other members of the board, based on our needs and the qualities required for the board of directors, including ethical character, integrity and maturity of judgment of the candidates; the level of experience of the candidates, their ideas regarding the material aspects of our business, the expertise of the candidates in fields relevant to us while complementing the training and experience of the other members of the board of directors; the will and ability of the candidates to devote the necessary time to their duties to the board of directors and its committees, the will of the candidates to serve on the board of directors for numerous consecutive financial periods and finally, the will of the candidates to refrain from engaging in activities which conflict with the responsibilities and duties of a director. The board researches the training and qualifications of potential new directors which seem to correspond to the selection criteria of the board of directors and, depending on the results of said research, organizes meetings with the potential candidates.
In the case of incumbent directors whose terms of office are set to expire, the board will review such directors’ overall service to us during their term of office, including the number of meetings attended, level of participation, quality of performance and any transactions of such directors with us during their term of office.
We may use various sources in order to identify the candidates for the board of directors, including our own contacts and the references of other directors, officers, advisors and executive placement agencies. We will consider director candidates recommended by shareholders and will evaluate those director candidates in the same manner in which we evaluate candidates recommended by other sources. In making recommendations for director nominees for the annual meeting of shareholders, we will consider any written recommendations of director candidates by shareholders received by our corporate secretary not later than 120 days before the anniversary of the previous year’s annual meeting of shareholders. Recommendations must include the candidate’s name, contact information and a statement of the candidate’s background and qualifications, and must be mailed to us. Following the selection of the candidates by the board of directors, we will propose a list of candidates to the shareholders, for our annual meeting of shareholders.
| 98 |
The board of directors does not have a nominating committee and has not adopted any formal written director term limit policy. Proposed nominations of director candidates are evaluated by our GHR committee.
GHR Committee
The mandate of the GHR committee consists of the evaluation of the proposed nominations of senior executives and director candidates to our board of directors, recommending for board approval, if appropriate, revisions of our corporate governance practices and procedures, developing new charters for any new committees established by the board of directors, monitoring relationships and communication between management and the board of directors, monitoring emerging best practices in corporate governance and oversight of governance matters and assessing the board of directors and its committees. The GHR committee is also in charge of establishing the procedure which must be followed by us to comply with applicable guidelines of the TSXV and NASDAQ Stock Market regarding corporate governance.
The GHR committee has the responsibility of evaluating the compensation, performance incentives as well as the benefits granted to our upper management in accordance with their responsibilities and performance as well as to recommend the necessary adjustments to our board of directors. The GHR committee also reviews the amount and method of compensation granted to the directors. The GHR committee may retain an external firm in order to assist it during the execution of its mandate. The GHR committee considers time commitment, comparative fees and responsibilities in determining compensation.
The GHR committee is composed of independent members within the meaning of NI 52-110 and NASDAQ Stock Exchange rules, namely Mr. Olds, Dr. Carter and Mr. Canan.
Periodic Assessments
The board of directors, its committees and each director are subject to periodic evaluations of their efficacy and contribution. The evaluation procedure consists in identifying any shortcomings and implementing adjustments proposed by directors at the beginning and during meetings of the board of directors and of each of its committees. Among other things, these adjustments deal with the level of preparation of directors, management and consultants employed by us, the relevance and sufficiency of the documentation provided to directors and the time allowed to directors for discussion and debate of items on the agenda.
Director Term Limits
The board actively considers the issue of term limits from time to time. At this time, the board does not believe that it is in our best interests to establish a limit on the number of times a director may stand for election. While such a limit could help create an environment where fresh ideas and viewpoints are available to the board, a director term limit could also disadvantage us through the loss of the beneficial contribution of directors who have developed increasing knowledge of, and insight into, us and our operations over a period of time. As we operate in a unique industry, it is difficult to find qualified directors with the appropriate background and experience and the introduction of a director term limit would impose further difficulty.
Policies Regarding the Representation of Women on the Board and Among Executive Officers
We have not adopted a formal written policy regarding diversity amongst executive officers and members of the board of directors, including mechanisms for board renewal, in connection with, among other things, the identification and nomination of women directors. Nevertheless, we recognize that gender diversity is a significant aspect of diversity and acknowledges the important role that women with appropriate and relevant skills and experience can play in contributing to the diversity of perspective on the board of directors.
| 99 |
Rather than considering the level of representation of women for directorship and executive officer positions when making board or executive officer appointments, we consider all candidates based on their merit and qualifications relevant to the specific role. While we recognize the benefits of diversity at all levels within its organization, we do not currently have any targets, rules or formal policies that specifically require the identification, consideration, nomination or appointment of candidates for directorship or executive management positions or that would otherwise force the composition of our board of directors and executive management team. Currently, we have one women director who is also our CEO.
| Item 14. | Principal Accounting Fees and Services |
Audit Fees
“Audit fees” consist of fees for professional services for the audit of our annual financial statements, interim reviews and consultations on accounting issues. KPMG LLP, our external auditors, billed CAD$308,160 for audit fees for the fiscal year ended March 31, 2020 and CAD$403,500 for audit fees for the fiscal year ended March 31, 2019. Audit fees for the fiscal year ended March 31, 2019 include fees related to securities filings.
Audit-Related Fees
“Audit-related fees” consist of fees for professional services that are reasonably related to the performance of the audit or review of our financial statements and which are not reported under “Audit Fees” above. KPMG LLP billed CAD$82,390 for the fiscal year ended March 31, 2020 and CAD$53,000 for the fiscal year ended March 31, 2019. Audit-related fees include French translation services. for the fiscal year ended March 31, 2019. Audit-Related fees for the fiscal year ended March 31, 2020 include fees related to securities filings.
Tax Fees
“Tax fees” consist of fees for professional services for tax compliance, tax advice and tax planning. KPMG LLP billed CAD$46,660 for tax fees for fiscal year ended March 31, 2020 and CAD$28,100 for tax fees for fiscal year ended March 31, 2019. Tax fees include, but are not limited to, preparation of tax returns.
All Other Fees
“Other fees” include all other fees billed for professional services other than those mentioned hereinabove. KPMG LLP billed no fees under this category for the fiscal years ended March 31, 2020 and March 31, 2019.
Pre-Approval Policies and Procedures
The audit committee approves all audit, audit-related services, tax services and other non-audit related services provided by the external auditors in advance of any engagement. Under the Sarbanes-Oxley Act of 2002, audit committees are permitted to approve certain fees for non-audit related services pursuant to a de minimus exception prior to the completion of an audit engagement. Non-audit related services satisfy the de minimus exception if the following conditions are met:
| · | the aggregate amount of all non-audit services that were not pre-approved is reasonably expected to constitute no more than five per cent of the total amount of fees paid by us and our subsidiaries to our external auditors during the fiscal year in which the services are provided; |
| · | we or our subsidiaries, as the case may be, did not recognize the services as non-audit services at the time of the engagement; and |
| · | the services are promptly brought to the attention of the audit committee and approved, prior to the completion of the audit, by the audit committee or by one or more of its members to whom authority to grant such approvals had been delegated by the audit committee. |
None of the services described above under “Principal Accounting Fees and Services” were approved by the audit committee pursuant to the de minimus exception.
| 100 |
| Item 15. | Exhibits, Financial Statement Schedules |
(a)(1) Financial Statements—The financial statements included in Item 8 are filed as part of this annual report on Form 10-K.
(a)(2) Financial Statement Schedules—All schedules have been omitted because they are not applicable or required, or the information required to be set forth therein is included in the consolidated Financial Statements or notes thereto included in Item 8 of this annual report on Form 10-K.
(a)(3) Exhibits—The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b) Exhibits—The exhibits listed on the Exhibit Index below are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
EXHIBITS INDEX
| 101 |
| 102 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: June 29, 2020
| ACASTI PHARMA INC. | ||||
| By: | /s/ Janelle D’Alvise | |||
| Name: Janelle D’Alvise | ||||
| Title: President and Chief Executive Officer and Director (Principal Executive Officer) | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Janelle D’Alvise | President and Chief Executive Officer and Director | June 29, 2020 | ||
| Janelle D’Alvise | (Principal Executive Officer) | |||
| /s/ Jean-François Boily | Vice President, Finance | June 29, 2020 | ||
| Jean-François Boily | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Dr. Roderick N. Carter | Director | June 29, 2020 | ||
| Dr. Roderick N. Carter | ||||
| /s/ Jean-Marie (John) Canan | Director | June 29, 2020 | ||
| Jean-Marie (John) Canan | ||||
| /s/ Donald Olds | Director | June 29, 2020 | ||
| Donald Olds |
| 101 |
Consolidated Financial Statements of
Acasti pharma inc.
For the years ended March 31, 2020 and 2019
F-1
| Acasti pharma inc. |
| Consolidated Financial Statements |
| For the years ended March 31, 2020 and 2019 |
F-2

| KPMG LLP | Telephone | (514) 840-2100 | |
| 600 de Maisonneuve Blvd. West | Fax | (514) 840-2187 | |
| Suite 1500, Tour KPMG | Internet | www.kpmg.ca Montréal (Québec) H3A 0A3 | |
| Canada |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Acasti Pharma Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Acasti Pharma Inc. (the Corporation) as of March 31, 2020 and 2019, the related consolidated statements of loss, comprehensive loss, shareholders’ equity, and cash flows for each of the years in the two-year period ended March 31, 2020, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Corporation as of March 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2020, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Corporation will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Corporation has incurred operating losses and negative cash flows from operations since its inception, and additional funds will be needed in the future that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Change in Accounting Framework and Reporting Currency
As discussed in Notes 2 and 21 to the consolidated financial statements, the Corporation has retrospectively adopted U.S. generally accepted accounting principles. Comparative figures, which were previously prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, have been adjusted as necessary.
As discussed in Note 2 to the consolidated financial statements, the Corporation has changed its reporting currency from Canadian dollars to U.S. dollars. The change in reporting currency has been applied retrospectively in the consolidated financial statements.
Basis for Opinion
These consolidated financial statements are the responsibility of the Corporation’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Corporation in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
F-3
KPMG LLP is a Canadian limited liability partnership and a member firm of the KPMG network of independent member firms affiliated with KPMG International Cooperative ("KPMG International"), a Swiss entity.
KPMG Canada provides services to KPMG LLP.

Page 2
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Corporation is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Corporation’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Corporation’s auditor since 2009.
Montréal, Québec
June 29, 2020
*CPA auditor, CA, public accountancy permit No. A122596
F-4
| Acasti pharma inc. |
| Consolidated Balance Sheets |
(Expressed in thousands of U.S. dollars except share data) |
| March 31, 2020 | March 31, 2019 | |||||||||
| (notes 2 and 21) | ||||||||||
| Notes | $ | $ | ||||||||
| Assets | ||||||||||
Current assets: | ||||||||||
| Cash and cash equivalents | 14,240 | 16,871 | ||||||||
| Short- term investments | 5 | - | 8,888 | |||||||
| Receivables | 4 | 546 | 1,189 | |||||||
| Other assets | 6 | 195 | 49 | |||||||
| Deferred financing costs | 13(b) | 121 | 134 | |||||||
| Prepaid expenses | 977 | 835 | ||||||||
| Total current assets | 16,079 | 27,966 | ||||||||
| Investments | 5 | - | 20 | |||||||
| Other assets | 6 | 473 | 417 | |||||||
| Equipment | 8 | 1,910 | 2,107 | |||||||
| Right of Use Asset | 147 | - | ||||||||
| Intangible assets | 9 | 4,244 | 6,386 | |||||||
| Total assets | 22,853 | 36,896 | ||||||||
| Liabilities and Shareholders’ equity | ||||||||||
| Current liabilities: | ||||||||||
| Trade and other payables | 10 | 7,319 | 12,307 | |||||||
| Unsecured convertible debentures | 12 | - | 1,361 | |||||||
| Lease liability | 76 | - | ||||||||
| Total current liabilities | 7,395 | 13,668 | ||||||||
| Derivative warrant liabilities | 11, 13(d)(e) | 2,393 | 12,183 | |||||||
| Lease Liability | 71 | - | ||||||||
| Total liabilities | 9,859 | 25,851 | ||||||||
Shareholders’ Equity: | ||||||||||
| Common shares | 13 | 137,424 | 110,857 | |||||||
| Additional paid-in capital | 13 | 9,797 | 8,150 | |||||||
| Accumulated other comprehensive loss | (7,887 | ) | (7,135 | ) | ||||||
| Accumulated deficit | (126,340 | ) | (100,827 | ) | ||||||
| Total Shareholder’s equity | 12,994 | 11,045 | ||||||||
Commitments and contingencies | 20 | |||||||||
| Total liabilities and shareholders’ equity | 22,853 | 36,896 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
| Acasti pharma inc. |
| Consolidated Statements of Loss and Comprehensive Loss |
(Expressed in thousands of U.S. dollars except share data) |
| Year ended | Year ended | |||||||||
| March 31, 2020 | March 31, 2019 | |||||||||
| (notes 2 and 21) | ||||||||||
| Notes | $ | $ | ||||||||
| Operating Expenses | ||||||||||
| Research and development expenses, net of government assistance | 7 | (15,974 | ) | (29,373 | ) | |||||
| General and administrative expenses | (5,799 | ) | (4,539 | ) | ||||||
| Sales and marketing | (2,665 | ) | (494 | ) | ||||||
| Loss from operations | (24,438 | ) | (34,406 | ) | ||||||
| Financial expenses | 13 (d)(e), 14 | (1,075 | ) | (4,960 | ) | |||||
| Net loss and total comprehensive loss | (25,513 | ) | (39,366 | ) | ||||||
Basic and diluted loss per share | 16 | (0.30 | ) | (0.73 | ) | |||||
Weighted average number of shares outstanding | 84,581,764 | 54,290,295 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-6
| Acasti pharma inc. |
| Consolidated Statements of Changes in Shareholders’ Equity |
(Expressed in thousands of U.S. dollars except share data) |
| Common Shares | ||||||||||||||||||||||||||
| Notes | Number | Dollar $ | Additional Paid-in Capital $ | Accumulated other comprehensive loss $ | Deficit $ | Total $ | ||||||||||||||||||||
| Balance, March 31, 2019 | 2, 21 | 78,132,734 | 110,857 | 8,150 | (7,135 | ) | (100,827 | ) | 11,045 | |||||||||||||||||
| Net loss and total comprehensive loss for the period | - | - | - | - | (25,513 | ) | (25,513 | ) | ||||||||||||||||||
| Cumulative translation adjustment | - | - | - | (752 | ) | - | (752 | ) | ||||||||||||||||||
| Warrants exercised | 11, 14 | 7,056,103 | 18,810 | (262 | ) | - | - | 18,548 | ||||||||||||||||||
| Net proceeds from shares issued under the at-the-market (ATM) program | 13(b) | 4,065,986 | 6,941 | - | - | - | 6,941 | |||||||||||||||||||
| Shares issued as a settlement | 10 | 900,000 | 738 | - | - | - | 738 | |||||||||||||||||||
| Stock based compensation | 54,626 | 78 | 1,909 | - | - | 1,987 | ||||||||||||||||||||
| Balance at March 31, 2020 | 90,209,449 | 137,424 | 9,797 | (7,887 | ) | (126,340 | ) | 12,994 | ||||||||||||||||||
| Common Shares | ||||||||||||||||||||||||||
| Notes | Number | Dollar $ | Additional Paid-in Capital $ | Accumulated other comprehensive loss $ | Deficit $ | Total $ | ||||||||||||||||||||
| Balance, March 31, 2018 | 2, 21 | 25,638,215 | 67,806 | 7,152 | (6,304 | ) | (61,461 | ) | 7,193 | |||||||||||||||||
| Net loss and total comprehensive loss for the period | (39,366 | ) | (39,366 | ) | ||||||||||||||||||||||
| Cumulative translation adjustment | (831 | ) | (831 | ) | ||||||||||||||||||||||
| Public offering | 13(c)(d) | 51,612,000 | 41,609 | 221 | 41,830 | |||||||||||||||||||||
| Warrants exercised | 17(b) | 772,474 | 1,345 | 1,345 | ||||||||||||||||||||||
| Stock based compensation | 4,167 | 2 | 777 | 779 | ||||||||||||||||||||||
| Issuance of shares for payment of interest on convertible debentures | 13(g) | 105,878 | 95 | 95 | ||||||||||||||||||||||
| Balance at March 31, 2019 | 78,132,734 | 110,857 | 8,150 | (7,135 | ) | (100,827 | ) | 11,045 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-7
| Acasti pharma inc. |
Consolidated Statements of Cash Flows |
(Expressed in thousands of U.S. dollars except share data) |
| Year Ended | Year Ended | |||||||||
| March 31, 2020 | March 31, 2019 | |||||||||
| (notes 2 and 21) | ||||||||||
| Notes | $ | $ | ||||||||
| Cash flows used in operating activities: | ||||||||||
| Net loss for the year | (25,513 | ) | (39,366 | ) | ||||||
| Adjustments: | ||||||||||
| Amortization of intangible assets | 9 | 1,910 | 1,949 | |||||||
| Depreciation of equipment | 8 | 410 | 384 | |||||||
| Stock-based compensation expense | 15 | 1,953 | 777 | |||||||
| Fair value of warrant liabilities | 11 | 1,116 | 4,745 | |||||||
| Accretion of interest on convertible debenture | 145 | 156 | ||||||||
| Unrealized exchange loss | 246 | 380 | ||||||||
| Total adjustments | 5,780 | 8,391 | ||||||||
| Changes in non-cash working capital items | 17 | (2,993 | ) | 6,294 | ||||||
| Changes in other assets | (225 | ) | 28 | |||||||
| Changes in deferred financing costs | 7 | (134 | ) | |||||||
| Net cash used in operating activities | (22,944 | ) | (24,787 | ) | ||||||
| Cash flows from (used in) investing activities: | ||||||||||
| Acquisition of equipment | 10, 17 | (319 | ) | (534 | ) | |||||
| Acquisition of short-term investments | (1,923 | ) | (18,850 | ) | ||||||
| Maturity of short-term investments | 10,380 | 9,942 | ||||||||
| Net cash from (used in) investing activities | 8,138 | (9,442 | ) | |||||||
| Cash flows from (used in) financing activities: | ||||||||||
| Net proceeds from shares issued under the at-the-market (ATM) program | 6,981 | - | ||||||||
| Net proceeds from public offering | 13(c)(d)(e)(f) | - | 44,892 | |||||||
| Proceeds from exercise of warrants | 7,706 | 796 | ||||||||
| Proceeds from exercise of stock options | 45 | 2 | ||||||||
| Payment of convertible debenture | (1,556 | ) | - | |||||||
| Net cash from financing activities | 13,176 | 45,690 | ||||||||
| Effect of exchange rate fluctuations on cash and cash equivalents | (254 | ) | 29 | |||||||
| Translation effect on cash and cash equivalents related to reporting currency | (747 | ) | (1,042 | ) | ||||||
| Net (decrease) increase in cash and cash equivalents | (2,631 | ) | 10,448 | |||||||
| Cash and cash equivalents, beginning of year | 16,871 | 6,423 | ||||||||
| Cash and cash equivalents, end of year | 14,240 | 16,871 | ||||||||
| Cash and cash equivalents are comprised of: | ||||||||||
| Cash | 4,869 | 1,420 | ||||||||
| Cash equivalents | 9,371 | 15,451 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-8
| Acasti pharma inc. |
Notes to the Consolidated Financial Statements |
(Expressed in thousands of U.S. dollars except share data) |
| 1. | Nature of Operations |
Acasti Pharma Inc. (“Acasti” or the “Corporation”) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 545, Promenade du Centropolis, Laval, Québec, H7T 0A3. In December 2019, Acasti incorporated a new wholly owned subsidiary named Acasti Innovation AG (“AIAG”) under the laws of Switzerland for the purpose of future development of the Corporation’s intellectual property.
The Corporation is subject to a number of risks associated with its ongoing priorities, including the conduct of its clinical program and its results, the establishment of strategic alliances and the development of new pharmaceutical products and their marketing. The Corporation’s current product in development, CaPre, requires approval from the U.S Food and Drug Administration and equivalent regulatory organizations in other countries before its sale can be authorized. Certain risks have been reduced for the longer term with the outcome of the Corporation’s actions, including the scale up of manufacturing of CaPre to 20 tons to support commercial launch, expansion of market development activities, and its intellectual property strategy execution with filed patent applications in more than 20 jurisdictions, with more than 20 issued patents and with numerous additional patent applications pending.
The Corporation has incurred significant operating losses and negative cash flows from operations since inception. To date, the Corporation has financed its operations through the public offering and private placement of Common Shares, units consisting of Common Shares and warrants, and convertible debt, the proceeds from research grants and research tax credits, and the exercises of warrants, rights and options. To achieve the objectives of its business plan, Acasti plans to raise the necessary funds through additional securities offerings and the establishment of strategic alliances as well as additional research grants and research tax credits. The ability of the Corporation to complete the needed financing and ultimately achieve profitable operations is dependent on a number of factors outside of the Corporation’s control.
| 2. | Summary of significant accounting policies |
Adoption of U.S. GAAP
The consolidated financial statements of the Corporation have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”). Comparative figures, which were previously presented in accordance with International Financial Reporting Standards (”IFRS”) as issued by the International Accounting Standards Board, have been adjusted as required to be compliant with the Corporation’s accounting policies under U.S. GAAP and are described in note 21.
Basis of presentation
These consolidated financial statements of Acasti Pharma Inc., which include the accounts of its subsidiary have been prepared in accordance with U.S. GAAP. All intercompany transactions and balances are eliminated on consolidation.
Going concern uncertainty:
The following summarizes the principal conditions or events relevant to the Corporation’s going concern assessment, which primarily considers the period of one year from the issuance date of these financial statements. The Corporation has incurred operating losses and negative cash flows from operations since its inception. The Corporation’s current assets of $16.1 million as at March 31, 2020 include cash and cash equivalents totaling $14.2 million. The Corporation’s current liabilities total $7.4 million at March 31, 2020 and are comprised primarily of amounts due to or accrued for creditors. Management projects that assuming positive Phase 3 results, additional funds will be needed in the future for us to file an NDA to obtain FDA approval for CaPre in the United States, to further scale up our manufacturing capabilities, and to complete marketing and other pre-commercialization activities. The Corporation’s plans include raising additional capital through additional securities offerings, as well as non-dilutive sources of capital such as grants or loans and strategic alliances, but there can be no assurance as to when or whether Acasti will complete any financings or strategic alliances. In particular, raising additional equity capital is subject to market conditions not within the Corporation’s control. If the Corporation does not raise additional funds or find one or more strategic partners, it may not be able to realize its assets and discharge its liabilities in the normal course of business. The Corporation currently has no arranged sources of financing other than its “At-the-market” sales agreement which provides for only conditional selling of the Corporation’s shares.
As a result, there is a substantial doubt about the Corporation’s ability to continue as a going concern.
F-9
| 2. | Summary of significant accounting policies (continued): |
Going concern uncertainty (continued):
The consolidated financial statements have been prepared on a going concern basis, which assumes the Corporation will continue its operations in the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the ordinary course of business. These consolidated financial statements do not include any adjustments to the carrying values and classification of assets and liabilities and reported expenses that might result from the outcome of this uncertainty and that may be necessary if the going concern basis was not appropriate for these consolidated financial statements. If the Corporation was unable to continue as a going concern, material impairment of the carrying values of the Corporation’s assets, including the intangible asset, could be required.
Significant accounting policies, estimates and judgments:
The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities (note 13) and stock-based compensation (note 15). Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and developments expenditures at each reporting date, are determining which research and development expenses qualify for research and development tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and, therefore, could be different from the amounts recorded.
Functional and reporting currency:
Effective March 31, 2020, the consolidated financial statements reporting currency has changed from Canadian dollars to U.S dollars. This change in reporting currency has been applied retrospectively such that all amounts are expressed in the consolidated financial statements of the Corporation and the accompanying notes thereto are expressed in thousands of U.S dollars, except for per share data. References to “$” are U.S dollars and references to “CAD $” are to Canadian dollars. For comparative purposes, historical consolidated financial statements were recast in U.S. dollars by translating assets and liabilities at the closing exchange rate in effect at the end of the respective period, expenses and cash flows at the average exchange rate in effect for the respective period and equity transactions at historical exchange rates. Translation gains and losses from the application of the U.S. dollar as the reporting currency while the Canadian dollar is the functional currency are included as part of the cumulative foreign currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive loss.
The Corporation’s functional currency is the Canadian dollar. The effects of exchange rate fluctuations on translating foreign currency monetary assets and liabilities into Canadian dollars are included in the statement of loss and comprehensive loss as foreign exchange gain/loss. Expense transactions are translated into the U.S. dollar reporting currency at the average exchange rate during the period, and assets and liabilities are translated at end of period exchange rates, except for equity transactions, which are translated at historical exchange rates.
Cash and Cash Equivalents:
Cash and cash equivalents comprise cash balances and highly liquid investments purchased with original maturities of three months or less. Cash and cash equivalents consist of term deposits, commercial papers, promissory notes and bankers’ acceptances held at the bank and recorded at cost, which approximates fair value.
Investments:
The Corporation’s investments consists of guaranteed investment certificates, term deposits and treasury bills and are classified as held-to-maturity securities. These investments are recorded at amortized cost. Investments with original maturities exceeding three months and less than one year are categorized as short-term.
Receivables:
Receivables are classified at amortized cost and recorded at the outstanding amount net of any provisions for uncollectible amount.
Deferred Financing Costs:
Deferred financing costs consists of fees charged by underwriters, attorneys, accountants, and other fees directly attributable to future issuances of shares. Provided these costs are determined to be recoverable, these costs are deferred and charged subsequently against the gross proceeds of the related equity transaction when it occurs. If at such time, the Corporation deems that these costs are no longer recoverable, they will be expensed as a component of finance expenses.
F-10
| 2. | Summary of significant accounting policies (continued): |
Equipment:
(i) Recognition and measurement:
Equipment is measured at cost less accumulated depreciation and accumulated impairment losses, if any.
Cost includes expenditures that are directly attributable to the acquisition of the asset, including all costs incurred in bringing the asset to its present location and condition.
Purchased software that is integral to the functionality of the related equipment is capitalized as part of that equipment.
Gains and losses on disposal of equipment are determined by comparing the proceeds from disposal with the carrying amount of equipment and are recognized net within operating expenses in the Consolidated Statement of Loss and Comprehensive Loss.
(ii) Subsequent costs:
The costs of the day-to-day servicing of equipment are recognized in profit or loss as incurred.
(iii) Depreciation:
Depreciation is recognized in profit or loss on either a straight-line basis or a declining basis over the estimated useful lives of each part of an item of equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. Items of equipment are depreciated from the date that they are available for use or, in respect of assets not yet in service, from the date they are ready for their intended use.
The estimated useful lives and rates for the current and comparative periods are as follows:
| Assets | Method | Period/Rate | ||||
| Furniture and office equipment | Declining balance | 20% | to | 30% | ||
| Computer equipment | Declining balance | 30% | ||||
| Laboratory equipment | Declining balance | 30% | ||||
| Production equipment | Declining balance | 10% | to | 30% | ||
Depreciation methods, useful lives and residual values are reviewed periodically and adjusted prospectively if appropriate.
Intangible assets:
Intellectual property and licenses that are acquired by the Corporation from a third party are capitalized and subsequently measured at cost less accumulated amortization and accumulated impairment losses, if they have finite useful lives, they are for approved products or if there are alternative future uses.
Amortization group
Amortization is calculated over the cost of the intangible asset less its residual value. Amortization is recognized in profit or loss on a straight-line basis over the estimated useful lives of intangible assets from the date that they are available for use, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. The estimated useful lives for the current and comparative periods are as follows:
| Assets | Period (years) | ||
| Patents | 20 | ||
| License | 8 | to | 14 |
Subsequent expenditure:
Subsequent expenditure is capitalized only when it increases the future economic benefits embodied in the specific asset to which it relates. All other expenditures, including expenditure on internally generated goodwill and brands, are recognized in profit or loss as incurred.
F-11
| 2. | Summary of significant accounting policies (continued): |
Research and Development Costs
Research and developments expenditures are expensed as incurred. These costs primarily consist of employees’ salaries and benefits related to research and development activities, consultants that conduct the Corporation’s clinical trials, independent auditors and consultants to perform investigation activities on behalf of the Corporation, clinical trial materials, stock-based compensation expense, and other non-clinical costs and regulatory approvals. Advance payments for goods and services that will be used in future research and development are recognized in prepaids or other assets and are expensed when the services are performed, or the goods are used.
Impairment of Long-Lived Assets:
The Corporation reviews the recoverability of its long-lived assets whenever events or changes in circumstances indicate that it is carrying amount may not be recoverable. The carrying amount is first compared with the undiscounted cash flows. If the carrying amount is higher than the sum of undiscounted cash flows, then the Corporation determines the fair value of the underlying asset group. Any impairment loss to be recognized is measured as the difference by which the carrying amount of the asset group exceeds the estimated fair value of the asset group. No such impairment has occurred in the years ended March 31, 2020 and 2019.
Stock based compensation:
The Corporation has in place a stock option plan for directors, officers, employees and consultants of the Corporation, with grants under the stock option plan approved by the Corporation’s Board of Directors. The plan provides for the granting of options to purchase Common Shares and the exercise price of each option equals the closing trading price of Common Shares on the day prior to the grant. The terms and conditions for acquiring and exercising options are set by the Corporation’s Board of Directors in accordance with and subject to the terms and conditions of the stock option plan. The Corporation measures the cost of such awards based on the fair value of the award at grant date, net of estimated forfeiture, and recognizes stock based compensation expense in the Consolidated Statements of Loss and Comprehensive Loss on a graded vesting basis over the requisite service period. The requisite service period equals the vesting periods of the awards. The fair value of options is estimated for each tranche of an award that vests on a graded basis. The fair value of options is estimated using the Black-Scholes option pricing model, which uses various inputs including estimated fair value of the Common Shares at the grant date, expected term, estimated volatility, risk-free interest rate and expected dividend yields of the Common Shares. The Corporation applies an estimated forfeiture rate derived from historical employee termination behaviour. If the actual forfeitures differ from those estimated by management, adjustment to compensation expense may be required in future periods.
Non-employee stock-based compensation transactions in which the Corporation receives goods or services as consideration for its own equity instruments are accounted for as stock-based compensation transactions. In June 2018, FASB issued Accounting Standards Update No. 2018-07, Improvements to Nonemployee Share-Based Payment Accounting. The amendment establishes that nonemployee share-based payment awards within the scope of Topic 718 be measured at grant-date fair value of the equity instruments issued and makes other amendments to align non-employee accounting more with employee accounting. The amendments are effective for fiscal years beginning after December 15, 2018. Early adoption is permitted, and the Corporation elected to early adopt this policy on April 1, 2018. Therefore, the Corporation establishes the fair value at the grant date for non-employee awards and measures the fair value based on the fair value of equity instruments issued. The fair value of a non employee award is estimated using the Black-Scholes option pricing model, which uses various inputs including estimated fair value of the Common Shares at the grant date, contractual term, estimated volatility, risk-free interest rate and expected dividend yields of the Common Shares. Non-employee awards remain within the scope of Topic 718 unless they are modified after service has been rendered. There was no effect of adopting the amendments on opening retained earnings at April 1, 2018; refer to note 13(e) for additional information.
Contingencies
The Corporation records accruals for contingencies expected to be incurred in connection with a loss contingency when it is probable that a liability has been incurred and the amount can be reasonably estimated.
Government grants:
Government grants are recorded as a reduction of the related expense or cost of the asset acquired. Government grants are recognized when there is reasonable assurance that the Corporation has met the requirements of the approved grant program and there is reasonable assurance that the grant will be received.
Grants that compensate the Corporation for expenses incurred are recognized in profit or loss in reduction thereof on a systematic basis in the same years in which the expenses are recognized. Grants that compensate the Corporation for the cost of an asset are recognized in profit or loss on a systematic basis over the useful life of the asset.
F-12
| 2. | Summary of significant accounting policies (continued): |
Leases:
Adoption of Topic 842 (Leases)
On April 1, 2019, the Corporation adopted Topic 842. There was no material impact on the consolidated financial statement from adopting the new standard given the Corporation only had short term leases at the time of adoption and the Corporation elected to apply the short-term lease exemption.
Subsequent to April 1, 2019, at the inception of an arrangement, the Corporation determines whether the arrangement is or contains a lease based on the unique facts and circumstances present in the arrangement and in accordance with the guidance of ASC Topic 842 “Leases”.
Operating lease liabilities and their corresponding right-of-use assets are initially recorded based on the present value of lease payments over the expected remaining lease term. Certain adjustments to the right-of-use asset may be required for items such as incentives received. The interest rate implicit in lease contracts is typically not readily determinable. As a result, the Corporation utilizes its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Corporation could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment. The Corporation does not have financing leases.
The Corporation has elected not to recognize leases with an original term of one year or less on the balance sheet. The Corporation typically only includes an initial lease term in its assessment of a lease arrangement. Options to renew a lease are not included in the Corporation’s assessment unless there is reasonable certainty that the Corporation will renew. In the year ended March 31, 2020 the Corporation modified the lease for its lab facility and recognized a right of use asset and a corresponding lease liability of $147. The new lease is for a two-year term and it was discounted using an incremental borrowing rate of 8%. The undiscounted obligation is $80 per year. The Corporation’s lease expense is recognized in research and development expenses.
Income tax:
Income tax expense comprises current and deferred taxes. Current and deferred taxes are recognized in profit or loss except to the extent that they relate to items recognized directly in equity or in other comprehensive income.
Current tax is the expected tax payable or receivable on the taxable income or loss for the year, using tax rates enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
Deferred tax is recognized in respect of temporary differences between the carrying amounts (tax base) of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax assets and liabilities are measured at the tax rate expected to apply when the underlying asset or liability is realised (settled) based on the rates that are enacted at the reporting date. Deferred tax assets and liabilities are offset if the Corporation has the right to set off the amount owed by with the amount owed by the other party, the Corporation intends to set off and the offset right is enforceable at law. A deferred tax asset is recognized for unused tax losses and tax credits, reduced by a valuation allowance to the extent that it is more likely than not that some portion or all of the deferred tax asset will not be realized.
Earnings per share:
The Corporation presents basic and diluted earnings per share (EPS) data for its Common Shares. Basic EPS is calculated by dividing the profit or loss attributable to the holders of Common Shares by the weighted average number of Common Shares outstanding during the year. Diluted EPS is determined by adjusting the profit or loss attributable to the holders of Common Shares and the weighted average number of Common Shares outstanding adjusted for the effects of all dilutive potential Common Shares, which comprise warrants and share options granted to employees.
Segment reporting:
An operating segment is a component of the Corporation that engages in business activities from which it may earn revenues and incur expenses. The Corporation has one reportable operating segment: the development and commercialization of pharmaceutical applications of its patent portfolio and licensed rights for cardiovascular diseases. The majority of the Corporation’s assets are located in Canada, while one major production unit, with a carrying value of $1,510 (March 31, 2019 - $1,873), is located in France at a third-party contract manufacturing facility.
F-13
| 2. | Summary of significant accounting policies (continued): |
Convertible Debentures:
The unsecured convertible debentures that existed in the financial statements as at March 31, 2019, were fully paid at maturity in February 2020. The unsecured convertible debentures could have been converted to Common Shares at the option of the holder, and the number of shares to be issued was fixed. The embedded conversion option in the convertible debentures meet the criteria to not be separately accounted for as a derivative. The convertible debentures were separated into liability and equity components. The liability component was recognized initially at the fair value of a similar liability that does not have an equity conversion option. The equity component was recognized initially as the difference between the fair value of the financial instrument as a whole and the fair value of the liability component. Any directly attributable transaction costs were allocated to the liability and equity components in proportion to their initial carrying amounts. Subsequent to initial recognition, the liability component is measured at amortized cost using the effective interest method. The equity component of the convertible debt is not remeasured subsequent to initial recognition.
Derivative financial instruments:
The Corporation has issued warrants of which some are accounted for as liability-classified derivatives over its own equity. Derivatives are recognized initially at fair value; attributable transaction costs are recognized in profit and loss as incurred. Subsequent to initial recognition, derivatives are measured at fair value, and all changes in their fair value are recognized immediately in profit or loss as a component of financial expenses
Other equity instruments:
Warrants that do not meet the definition of a liability instrument are recognized in equity as additional paid in capital.
Fair Value Measurements
Certain of the Corporation’s accounting policies and disclosures require the determination of fair value, for both financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following methods.
Financial assets and liabilities:
In establishing fair value, the Corporation uses a fair value hierarchy based on levels as defined below:
| · | Level 1: defined as observable inputs such as quoted prices in active markets. |
| · | Level 2: defined as inputs other than quoted prices in active markets that are either directly or indirectly observable. |
| · | Level 3: defined as inputs that are based on little or no observable market data, therefore requiring entities to develop their own assumptions. |
The Corporation has determined that the carrying values of its short-term financial assets and liabilities (cash and cash equivalents, and trade and other payables) approximate their fair value given the short-term nature of these instruments. The fair value of the liability component of the convertible debenture is determined by discounting future cash flows using a rate that the Corporation could obtain for loans with similar terms, conditions and maturity dates. The fair value of this liability at March 31, 2019 approximates the carrying amount and was measured using level 3 inputs. The Corporation measured its derivative warrant liabilities at fair value on a recurring basis using level 3 inputs.
F-14
| 3. | Recent Accounting Pronouncements |
In June 2016, the FASB issued ASU 2016-13-Financial Instruments-Credit Losses (Topic 326), which amends guidance on reporting credit losses for assets held at amortized cost basis and available for sale debt securities. For assets held at amortized cost, the new guidance eliminates the probable initial recognition threshold in current GAAP and, instead, requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. ASU 2016-13 will affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, and any other financial assets not excluded from the scope that have the contractual right to receive cash. ASU 2016-13 is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2022. Management has not yet evaluated the impact of this ASU on the consolidated financial statements.
In August 2018, the FASB issued ASU 2018-15-Intangibles-Goodwill and Other-Internal-Use Software: Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract. ASU 2018-15 aligns the requirements for capitalizing implementation costs in such cloud computing arrangements with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. This ASU is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 and early adoption is permitted. Entities can choose to adopt the new guidance prospectively or retrospectively. Management has not yet evaluated the impact of this ASU on the consolidated financial statements.
| 4. | Receivables: |
| March 31, 2020 | March 31, 2019 | |||||||||
| Notes | $ | $ | ||||||||
| Sales tax receivables | 301 | 463 | ||||||||
| Government assistance | 7 | 209 | 652 | |||||||
| Interest receivable | 11 | 69 | ||||||||
| Other receivables | 25 | 5 | ||||||||
| Total receivables | 546 | 1,189 | ||||||||
| 5. | Investments: |
The Corporation holds various marketable securities with maturities greater than 3 months at the time of purchase as follows:
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Term deposits issued in US currency earning interest at 2.50% and maturing on various dates from April 8, 2019 to March 12, 2020 | - | 2,020 | ||||||
| Treasury bills issued in CAD currency earning interest at rates ranging from 1.83% to 1.90% and maturing on various dates from April 2, 2019 to July 25, 2019 | - | 6,888 | ||||||
| Total investments | - | 8,908 | ||||||
| Short-term investments | - | 8,888 | ||||||
| Investments | - | 20 | ||||||
F-15
| 6. | Other Assets |
The Corporation owns a reserve of krill oil in which amounts are expensed as it is used. The following table summarizes information regarding activities of amounts of the krill oil usage in the research and development production processes and for NKPL66 (the active pharmaceutical ingredient for CaPre) manufacturing.
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Balance – beginning of year | 466 | 513 | ||||||
| Purchased | 312 | 53 | ||||||
| Used | (90 | ) | (79 | ) | ||||
| Foreign exchange- translation effect | (20 | ) | (21 | ) | ||||
| Balance – end of year | 668 | 466 | ||||||
Current-other asset | 195 | 49 | ||||||
| Other asset | 473 | 417 | ||||||
F-16
| 7. | Government assistance: |
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Investment tax credit | 182 | 652 | ||||||
| Government grant | 27 | - | ||||||
| Total government assistance | 209 | 652 | ||||||
Government assistance is comprised of a government grant from the Canadian federal government and research and development investment tax credits receivable from the Quebec provincial government which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. For the years ended March 31, 2020 and 2019, the Corporation recorded $149 and $445, respectively, as a reduction of research and development expenses in the Consolidated Statements of Loss and Comprehensive Loss.
The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. Unrecognized Canadian federal tax credits may be used to reduce future Canadian federal income tax and expire as follows:
| $ | ||||
| 2029 | 8 | |||
| 2030 | 21 | |||
| 2031 | 32 | |||
| 2032 | 306 | |||
| 2033 | 314 | |||
| 2034 | 310 | |||
| 2035 | 369 | |||
| 2036 | 203 | |||
| 2037 | 224 | |||
| 2038 | 230 | |||
| 2039 | 234 | |||
| 2040 | 217 | |||
| 2,468 | ||||
In September 2019, the Corporation was awarded up to CAD $750,000 in non-dilutive and non-repayable funding from the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP) to apply towards eligible research and development disbursements of the Corporation’s unique commercial production platform for CaPre. As at March 31, 2020 the Corporation has claimed $164 in connection with this program, which has been recorded as a reduction of research and development expenses in the Consolidated Statements of Loss and Comprehensive Loss.
| 8. | Equipment: |
| March 31, 2020 | Cost | Accumulated depreciation | Net book value | |||||||||
| $ | $ | $ | ||||||||||
| Furniture and office equipment | 15 | 3 | 12 | |||||||||
| Computer equipment | 64 | 18 | 46 | |||||||||
| Laboratory equipment | 684 | 343 | 341 | |||||||||
| Production equipment | 2,341 | 830 | 1,511 | |||||||||
| 3,104 | 1,194 | 1,910 | ||||||||||
| March 31, 2019 | Cost | Accumulated depreciation | Net book value | |||||||||
| $ | $ | $ | ||||||||||
| Furniture and office equipment | 6 | 1 | 5 | |||||||||
| Computer equipment | 23 | 10 | 13 | |||||||||
| Laboratory equipment | 490 | 274 | 216 | |||||||||
| Production equipment | 2,438 | 565 | 1,873 | |||||||||
| 2,957 | 850 | 2,107 | ||||||||||
For the year ended March 31, 2020, depreciation expense was $410 (2019 $384) and was included in research and development expenses.
F-17
| 9. | Intangible assets: |
In 2009 and again in 2012, the Corporation entered into agreements with Neptune Wellness Solutions Inc. (Neptune) pursuant to which the Corporation obtained a license and exercised its option under this license agreement to pay in advance all of the future royalties payable to Neptune. This license allows the Corporation to exploit the intellectual property rights in order to develop novel active pharmaceutical ingredients into commercial products for the prescription drugs market. The license agreement, together with the Corporation-owned intellectual property, allows the “freedom to operate” for CaPre, which is currently the Corporation’s only prescription drug candidate in development. The Corporation believes that upon the expiry of the last licensed Neptune patent in 2022, the Corporation’s expanding patent portfolio will cover CaPre, and that it will not require any licenses to support the commercialization of CaPre.
| March 31, 2020 | Cost | Accumulated depreciation | Net book value | |||||||||
| $ | $ | $ | ||||||||||
| License | 18,025 | 13,781 | 4,244 | |||||||||
| March 31, 2019 | Cost | Accumulated depreciation | Net book value | |||||||||
| $ | $ | $ | ||||||||||
| License | 18,988 | 12,602 | 6,386 | |||||||||
Amortization expense on intangible assets for the years ended March 31, 2020 and 2019 was $1,910 and $1,949, respectively, and have been included in research and development expenses.
| 10. | Trade and other payables: |
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Trade payables | 1,713 | 3,047 | ||||||
| Accrued liabilities and other payables | 4,247 | 7,697 | ||||||
| Employee salaries and benefits payable | 1,359 | 825 | ||||||
| Legal settlement paid via common shares | - | 738 | ||||||
| Total trade and other payables | 7,319 | 12,307 | ||||||
On May 10, 2019, the Corporation announced the settlement regarding legal claims made by its former chief executive (“CEO”) officer with respect to the termination of his employment. Pursuant to the settlement agreement, the Corporation agreed to issue 900,000 common shares at CAD$1.10 per share to the former CEO. In addition, the Corporation agreed to reimburse the former CEO for legal fees of $52. Furthermore, pursuant to the settlement agreement, the Corporation receives a full and final release from the former CEO on all procedures in connection with the termination of his employment. This settlement was accrued as at March 31, 2019 and the expense of $790 was included as part of General and administrative expenses. The case is closed, and no further costs are expected.
F-18
| 11. | Derivative warrant liabilities: |
The warrants issued as part of the public offering of units composed of Common Shares and Common Share purchase warrants on May 9, 2018 and May 14, 2018 (see note 13) are derivative warrant liabilities given the warrant indenture contains certain contingent provisions that allow for cash settlement.
Warrants issued as part of a public offering of units composed of Common Shares and Common Share purchase warrants on December 27, 2017 are derivative warrant liabilities given the currency of the exercise price is different from the Corporation’s functional currency.
The derivative warrant liabilities are measured at fair value at each reporting period and the reconciliation of changes in fair value is presented in the following tables:
| Warrants issued May 2018 | Warrants issued December 27, 2017 | |||||||||||||||
| March 31, 2020 | March 31, 2019 | March 31, 2020 | March 31, 2019 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Balance – beginning of year | 6,177 | - | 6,005 | 4,987 | ||||||||||||
| Issued during the year | - | 3,323 | - | - | ||||||||||||
| Amount transferred to Equity | (6,072 | ) | (550 | ) | (4,770 | ) | - | |||||||||
| Change in fair value | 1,115 | 3,579 | 1 | 1,166 | ||||||||||||
| Translation effect | (35 | ) | (175 | ) | (28 | ) | (147 | ) | ||||||||
| Balance – end of year | 1,185 | 6,177 | 1,208 | 6,006 | ||||||||||||
| Fair value per warrant issuable | 0.17 | 0.62 | 0.18 | 0.63 | ||||||||||||
The fair value of the derivative warrant liabilities was estimated using the Black-Scholes option pricing model and based on the following assumptions:
| Warrant liabilities issued May 2018 | Warrant liabilities issued December 27, 2017 | |||||||||||||||
March 31, $ | March 31, $ | March 31, 2020 $ | March 31, 2019 $ | |||||||||||||
| Exercise price | CAD $1.31 | CAD $1.31 | USD $1.26 | USD $1.26 | ||||||||||||
| Share price | CAD $0.53 | CAD $1.35 | USD $0.38 | USD $1.02 | ||||||||||||
| Risk-free interest | 0.66 | % | 1.52 | % | 0.37 | % | 2.23 | % | ||||||||
| Contractual life (years) | 3.11 | 4.11 | 2.74 | 3.75 | ||||||||||||
| Expected volatility | 107 | % | 94.58 | % | 125.03 | % | 107.57 | % | ||||||||
The Corporation measured its derivative warrant liabilities at fair value on a recurring basis. These financial liabilities were measured using level 3 inputs (see Note 13).
As at March 31, 2020, the effect of an increase or a decrease of 5% of the volatility used, which is the significant unobservable input in the fair value estimate, would result in a loss of $167 or a gain of $173, respectively.
As at March 31, 2020, the effect of a 5% strengthening of the U.S. dollar against the Canadian dollar, would result in a loss of $88. An assumed 5% weakening of the U.S. dollar against the Canadian dollar would have an equal but opposite effect on the basis that all other variables remained constant.
F-19
| 12. | Unsecured convertible debentures |
Concurrently with the public offering described in note 14, on February 21, 2017, the Corporation issued $ 1,522 (CAD$ 2,000) aggregate principal amount of unsecured convertible debentures maturing February 21, 2020 and contingent warrants to acquire up to 1,052,630 Common Shares (the “Private Placement”). The debentures were paid in full at maturity.
The debentures could have been converted into Common Shares at any time by the holder at a fixed price of CAD $1.90 per Common Share except if the Corporation paid before the maturity, all or any portion of the convertible debentures. If the Corporation had paid all or any portion of the convertible debenture before maturity, then warrants would have become exercisable at CAD $1.90 per Common Share for the equivalent convertible debenture amount prepaid. The contingent warrants were exercisable for the remaining term of the convertible debt for the same price as the conversion options. The unsecured convertible debentures were issued at a discount of 3.5% to the principal amount, for aggregate gross proceeds of $1,469 (CAD $1,930).
The convertible debentures provided the Corporation an accelerated conversion right whereby the Corporation could have, at any time at least four months after the date of issuance of the convertible debentures, accelerate the conversion of the debentures to Common Shares in the event that the volume weighted average price of the Common Shares on the TSX Venture Exchange was equal to or exceeds CAD$2.65, subject to customary adjustment provisions, during 20 consecutive trading days.
The interest paid on the convertible debentures under the terms of the agreement was 8% per annum, payable on a quarterly basis in cash or Common Shares or a combination thereof, commencing on March 31, 2017. The decision to pay the interest due in cash or shares was at the discretion of the Corporation and the number of Common Shares to be issued were calculated at the current market price as at the close of business on the day before the interest payment was to be made. Payment in Common Shares would have been at a floor price of $0.10 per share, with the difference between the amount payable and the amount computed at floor price payable in cash.
The proceeds of the Private Placement were split between the liability and the equity at the time of the Private Placement. Both the conversion option and contingent warrants were considered the equity component of the Private Placement. The fair value of the liability component was determined through a discounted cash flow analysis using a discount rate of 20% that was set based on a similar debt and maturity considering the Corporation’s credit risk, excluding the conversion option and contingent warrants. The amount allocated to the equity component was the residual amount after deducting the fair value of the financial liability component from the fair value of the entire financial instrument. Subsequent to initial recognition, the liability was measured at amortized cost calculated using the effective interest rate method and accreted up to the principal balance at maturity. The interest accretion is presented in financial expenses. The equity component is not re-measured. Transaction costs were allocated to the components in proportion to their initial carrying amounts. The portion allocated to the liability was recognized as a reduction of the debt whereas the portion allocated to other equity was recognized as a reduction to other equity.
The split between the liability and equity component portions of the Private Placement are summarized below:
| Liability component | Equity component | Total Private Placement | |||||||||
| $ | $ | $ | |||||||||
| Balance at March 31, 2018 | 1,255 | 220 | 1,475 | ||||||||
| Effective interest for the year | 156 | - | 156 | ||||||||
Translation effect |
(87) | - | (87) | ||||||||
| Interest payable during the year | 37 | 37 | |||||||||
| Balance at March 31, 2019 | 1,361 | 220 | 1,581 | ||||||||
Accretion of interest on convertible debenture |
145 | - | 145 | ||||||||
| Translation effect | 50 | - | 50 | ||||||||
| Shares issued upon exercise of warrants | - | (33 | ) | (33 | ) | ||||||
| Payment upon maturity of debentures | (1,556 | ) | - | (1,556 | ) | ||||||
| Balance at March 31, 2020 | - | 187 | 187 | ||||||||
F-20
| 13. | Capital and other components of equity |
| (a) | Common Shares: |
Authorized capital stock:
Unlimited number of shares:
| Ø | Class A shares (Common Shares), voting (one vote per share), participating and without par value |
| Ø | Class B shares, voting (ten votes per share), non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class B shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class B shares are redeemable at the holder’s discretion for CAD $0.80 per share, subject to certain conditions. There are none issued and outstanding. |
| Ø | Class C shares, non-voting, non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class C shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class C shares are redeemable at the holder’s discretion for CAD $0.20 per share, subject to certain conditions. There are none issued and outstanding. |
| Ø | Class D and E shares, they are non-voting, non-participating, without par value and maximum monthly non-cumulative dividend between 0.5% and 2% on the amount paid per share. Class D and E shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class D and E shares are redeemable at the holder’s discretion, subject to certain conditions. There are none issued and outstanding. |
| (b) | “At-the-market” sales agreement |
On February 14, 2019, the Corporation entered into an “at-the-market” (ATM) sales agreement with B. Riley FBR, Inc. (“B. Riley”) pursuant to which the Common Shares may be sold from time to time for aggregate gross proceeds of up to $30 million, with sales only being made on the NASDAQ Stock Market. The Common Shares would be issued at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The ATM has a 3-year term and requires the Corporation to pay between 3% and 4% commission to B. Riley based on volume of sales made.
As at March 31, 2020, the Corporation sold a total of 4,065,986 Common Shares (none as at March 31, 2019) through the ATM program over the NASDAQ Stock Market, for net proceeds of $7 million (net of commissions paid for approximately $291). The shares were sold at the prevailing market prices which resulted in an average price of approximately $1.79 per share. In addition, a total of $40 of expenses originally recorded as deferred financing costs were reclassified to equity.
| (c) | Public Offerings – October 2018: |
On October 9, 2018, the Corporation closed a U.S. public offering of 16,600,000 Common Shares at a price of $1.00 per share. In addition, the underwriters fully exercised their over-allotment option to purchase 2,490,000 additional Common Shares at the same public offering price. This offering generated gross proceeds of $19.1 million (CAD $24.7 million), which resulted in net proceeds to the Corporation of $17.4 million (CAD $22.6 million) and a total of 19,090,000 Common Shares issued.
On October 23, 2018, the Corporation closed a Canadian public offering of 18,750,000 Common Shares at a price of CAD $1.28 per share. In addition, the underwriters fully exercised their over-allotment option to purchase 2,812,500 additional Common Shares at the same public offering price. This offering generated gross proceeds of $21.1 million (CAD $27.6 million), which resulted in net proceeds to the Corporation of approximately $19.4 million (CAD $25.4 million) and a total of 21,562,500 Common Shares issued.
F-21
| 13. | Capital and other components of equity (continued): |
| (d) | Public Offering – May 2018: |
On May 9, 2018, the Corporation closed a Canadian public offering issuing 9,530,000 units at a price of CAD $1.05 per unit for gross proceeds of $7.8 million (CAD$10 million). The units issued consist of 9,530,000 Common Shares and 9,530,000 warrants. Each warrant entitles the holder thereof to acquire one Common Share at an exercise price of CAD $1.31 at any time until May 9, 2023.
On May 14, 2018, the underwriters exercised their over-allotment option by purchasing an additional 1,429,500 units at a price of CAD $1.05 per unit, for additional gross proceeds of $1.1 million (CAD $1.5 million). The units issued consist of 1,429,500 Common Shares and 1,429,500 warrants. Each Warrant entitles the holder thereof to acquire one Common Share of the Corporation at an exercise price of CAD $1.31 at any time until May 9, 2023.
At the time of issuance, the warrant component of these units are derivative warrant liabilities for accounting purposes due to certain contingent provisions that allow for cash settlement in the warrant agreement (see note 13). The proceeds of the offering are required to be split between the derivative warrant liabilities and the equity-classified Common shares at the time of issuance of the units. The fair value of the derivative warrant liabilities at the time of issuance was determined to be $3.3 million (CAD $4.3 million) and the residual of the proceeds of $4.8 million (CAD $6.2 million) were allocated to the Common Shares. Issuance costs related to this transaction totaled approximately $1.4 million (CAD $1.8 million) and have been allocated between the derivative warrant liabilities and Common shares based on relative value. Resulting from this allocation, $0.5 million (CAD $0.7 million) has been allocated to the derivative warrant liabilities and is recognized in finance expenses in the Statements of Loss and Comprehensive Loss, whereas the remaining portion of $0.9 million (CAD $1.1 million) in issuance costs was allocated to the Common Shares and recognized as a reduction to Common Shares, in the Balance Sheet.
The fair value of the public offering warrants at issuance was estimated using to the Black-Scholes option pricing model and was based on the following weighted average assumptions:
| May 2018 CAD | ||||
| Exercise price | $ | 1.31 | ||
| Share price | $ | 0.82 | ||
| Risk-free interest | 2.21 | % | ||
| Contractual life (years) | 5 | |||
| Expected volatility | 87.40 | % | ||
The weighted average fair value of the public offering warrants issued in May 2018 was determined to be $0.30 (CAD $0.39) per warrant. Changes in the subsequent measurement of fair value of the warrants are recognized in financial expenses.
As part of the transaction, the Corporation also issued broker warrants to purchase up to 547,975 Common Shares. Each broker warrant entitles the holder thereof to acquire one Common Share at an exercise price of CAD $1.05, at any time until May 9, 2023. The broker warrants are considered to be equity-classified non-employee stock-based awards and are thus accounted for at fair value at grant date and not subsequently revalued. To determine the fair value of these broker warrants, a Black-Scholes options pricing model was used based on the following assumptions:
| May 2018 CAD | ||||
| Exercise price | $ | 1.05 | ||
| Share price | $ | 0.81 | ||
| Risk-free interest | 2.20 | |||
| Contractual life (years) | 5 | |||
| Expected volatility | 87.40 | % | ||
The total value associated with the broker warrants amounted to $220 (CAD $283) and was recorded in additional paid in capital.
F-22
| 13. | Capital and Other Components of Equity (continued): |
| (e) | Public offering – December 27, 2017 |
On December 27, 2017, the Corporation closed a U.S. public offering of 9,900,990 units at a price of US$1.01 per unit for gross proceeds of $10 million. The units issued consist of 9,900,990 Common Shares and 8,910,891 warrants to purchase one Common Share. As part of this closing, the underwriters also partially exercised for nil consideration the over-allotment option for warrants, which were issued for a right to purchase 892,044 Common Shares at an exercise price of $1.26.
The warrants forming part of the units are derivative warrant liabilities for accounting purposes due to the currency of the exercise price being different from the Corporation’s functional currency. The proceeds of the offering are required to be split between the derivative warrant liabilities and the equity-classified Common Share at the time of issuance of the units. The fair value of the derivative warrant liabilities at the time of issuance was determined to be $4.7 million and the residual of the proceeds was allocated to the Common Shares. Total issuance costs related to this transaction totaled $2 million. The issuance costs have been allocated between the warrants and Common Shares based on relative value. The portion allocated to the warrants was recognized in financial expenses in the Statements of Loss and Comprehensive Loss, whereas the portion allocated to Common Shares was recognized as a reduction to Common Shares in the Balance Sheet.
At the time of issuance, the fair value of the warrants at issuance was estimated according to the Black-Scholes option pricing model and based on the following assumptions:
| December 27, 2017 | ||||
| Exercise price | $ | 1.26 | ||
| Share price | $ | 0.97 | ||
| Risk-free interest | 2.22 | % | ||
| Contractual life (years) | 5 | |||
| Expected volatility | 93.52 | % | ||
The fair value of the warrants issued was determined to be $0.47 per warrant as at December 27, 2017. Changes in the fair value of the warrants are recognized in financial expenses.
As part of the transaction, the Corporation also issued broker warrants to purchase up to 495,050 Common Shares. Each broker warrant entitles the holder thereof to acquire one Common Share at an exercise price of $1.2625, at any time until December 19, 2022. The broker warrants were considered derivative warrant liabilities at the time of the issuance, due to the currency of the exercise price being different from the Corporation’s functional currency. The fair value of the derivative warrant liabilities at the time of issuance was determined to be $321, (CAD $406) which was estimated according to the Black-Scholes option pricing model and based on the same assumptions as those used to value the warrants forming part of the units. Upon adoption of FASB Accounting Standards Update No. 2018-07, Improvements to Nonemployee Share-Based Payment Accounting on April 1, 2018, the broker warrants became equity-classified and the fair value as determined on April 1, 2018, was reclassified from derivative warrant liability to additional paid-in capital in the amount of $65. This amount is not subsequently remeasured. To determine the fair value of the broker warrants, a Black-Scholes option pricing model was used based on the following assumptions at the transition date to ASU No. 2018-07:
| April 1, 2018 | ||||
| Exercise price | $ | 1.2625 | ||
| Share price | $ | 1.02 | ||
| Risk-free interest | 2.56 | % | ||
| Remaining Contractual life (years) | 4.75 | |||
| Expected volatility | 95.16 | % | ||
F-23
| 13. | Capital and Other Components of Equity (continued): |
| (f) | Public offering - February 21, 2017: |
Concurrently with the private placement described in Note 12, on February 21, 2017, the Corporation closed a public offering of 3,930,518 units at a price of CAD $1.45 per unit for gross proceeds of $4,337 (CAD $5,699). Each unit consists of one Common Share and one half of one Common Share purchase warrant. Each whole warrant entitles the holder thereof to purchase one Common Share at an exercise price of CAD $2.15 per share, at any time until February 21, 2022. The transaction costs associated with the public offering amounted to $906 (CAD $1,190) and were allocated between Common Shares and additional paid-in capital.
As part of the transaction, the Corporation also issued broker warrants to purchase up to 234,992 Common Shares at an exercise price of CAD $2.15 per share. The total costs associated with the broker warrants amounted to $110 (CAD $144) and were allocated to additional paid-in capital (and reclassified to Common Shares upon exercise subsequent exercise of warrants).
The warrants issued as part of the units and the broker warrants include an “Acceleration Right”, related to the Corporation’s right to accelerate the expiry date of the warrants. The Acceleration Right clause means the right of the Corporation to accelerate the expiry date to a date that is not less than 30 days following delivery of the acceleration notice if, at any time at least four months after the effective date, the volume-weighted average trading price of the Common Shares equals or exceeds CAD $2.65 for a period of 20 consecutive trading days on the TSXV.
| (g) | Issuance of shares: |
The following table summarizes the shares issued to settle the payment of accrued interest on the unsecured convertible debentures with the corresponding amount recorded to Common Shares. Subsequent to September 30, 2018 to the settlement of the debentures, all scheduled interest payments were paid in cash.
|
Accrued interest as at |
Share issuance date |
Number of shares |
Amount CAD $ |
| March 31, 2017 | April 7, 2017 | 9,496 | 17 |
| June 30, 2017 | August 15, 2017 | 23,885 | 40 |
| September 30, 2017 | December 27, 2017 | 22,783 | 40 |
| December 31, 2017 | March 27, 2018 | 33,605 | 40 |
| March 31, 2018 | June 6, 2018 | 30,348 | 40 |
| June 30, 2018 | August 21, 2018 | 51,807 | 40 |
| September 30, 2018 | October 31, 2018 | 23,723 | 40 |
| 195,647 | 257 |
F-24
| 13. | Capital and other components of equity (continued): |
| (h) | Warrants: |
The warrants of the Corporation are composed of the following:
| March 31, 2020 | March 31, 2019 | |||||||||||||||
| Number outstanding |
Amount | Number outstanding |
Amount | |||||||||||||
| $ | $ | |||||||||||||||
| Liability | ||||||||||||||||
| May 2018 public offering warrants 2018 (i) | 6,593,750 | 1,146 | 10,188,100 | 6,178 | ||||||||||||
| Series December 2017 U.S. public offering warrants 2017 (ii) | 7,072,962 | 1,247 | 9,801,861 | 6,005 | ||||||||||||
| 13,666,712 | 2,393 | 19,989,961 | 12, 183 | |||||||||||||
| Equity | ||||||||||||||||
| Public offering warrants | ||||||||||||||||
| Public offering broker warrants May 2018 (iii) | 222,976 | 89 | 547,975 | 219 | ||||||||||||
| Public offering U.S. broker warrants December 2017 (iv) | 259,121 | 161 | 495,050 | 308 | ||||||||||||
| Public offering warrants February 2017 (v) | 1,723,934 | 631 | 1,904,034 | 697 | ||||||||||||
| Private Placement- contingent warrants | ||||||||||||||||
| 2017 unsecured convertible debenture conversion option and contingent warrants (vi) | - | - | 1,052,630 | 235 | ||||||||||||
| 2,206,031 | 881 | 3,999,689 | 1,459 | |||||||||||||
| (i) | Warrant to acquire one Common Share at an exercise price of CAD $1.31, expiring on May 9, 2023. |
| (ii) | Warrant to acquire one Common Share at an exercise price of $1.26, expiring on December 27, 2022. |
| (iii) | Warrant to acquire one Common Share o at an exercise price of CAD $1.05, expiring on May 9, 2023. |
| (iv) | Warrant to acquire one Common Share at an exercise price of $1.2625, expiring on December 19, 2022. |
| (v) | Warrant to acquire one Common Share at an exercise price of CAD $2.15, expiring on February 21, 2022. |
| (vi) | Warrant to acquire one Common Share at an exercise price of CAD $1.90, expiring on February 21, 2020, exercisable only for any portion of or all debentures paid by the Corporation prior to maturity. During the year ended March 31, 2020, convertible debentures were fully repaid with an amount of CAD $2 million resulting in the cancellation of the outstanding conversion option. |
Warrants exercised: During the year ending March 31, 2020, the following warrants were exercised with the resulting cash proceeds:
Number exercised | Proceeds $ | |||||||
| May 2018 over-allotment Warrants 2018 | 3,594,350 | 3,567 | ||||||
| Series December 2017 US Public offering Warrants 2017 | 2,676,611 | 3,373 | ||||||
| Public offering warrants February 2017 | 180,100 | 292 | ||||||
| Public offering Broker warrants May 2018 | 325,000 | 257 | ||||||
| Contingent warrants private placement 2017 | 150,000 | 217 | ||||||
| 6,926,061 | 7,706 | |||||||
F-25
| 13. | Capital and other components of equity (continued): |
| (h) | Warrants (continued): |
During the year ended March 31, 2020, 235,929 broker warrants and 52,288 derivative warrants offered as part of the December 2017 U.S. public offering were exercised on a cashless basis to acquire 136,013 Common Shares.
During the year ended March 31, 2019, 771,400 warrants offered as part of the May 2018 public offering were exercised at an exercise price of $1.31 per Common Share of the Company, resulting in $0.78 million of cash proceeds. In addition, 4,455 warrants offered as part of the December 2017 U.S. public offering were exercised in a cashless manner to acquire 1,074 Common Shares of the Company. A total of 772,474 Common Shares were issued as a result of 775,855 warrants being exercised.
| 14. | Financial expenses: |
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Foreign exchange gain (loss) | (2 | ) | 212 | |||||
| Interest payable on convertible debenture | (102 | ) | (122 | ) | ||||
| Accretion of interest on convertible debenture | (145 | ) | (156 | ) | ||||
| Financing costs | (46 | ) | (507 | ) | ||||
| Interest income | 336 | 358 | ||||||
| Change in fair value of warrant liabilities | (1,116 | ) | (4,745 | ) | ||||
| Financial (expenses) | (1,075 | ) | (4,960 | ) | ||||
F-26
| 15. | Stock based compensation: |
At March 31, 2020, the Corporation has the following stock-based compensation arrangement:
| (a) | Corporation stock option plan: |
The Corporation has in place a stock option plan for directors, officers, employees and consultants of the Corporation. An amendment of the stock option plan was approved by shareholders on August 27, 2019. The amendment provides for an increase to the existing limits for Common Shares reserved for issuance under the stock option plan. The stock option plan continues to provide for the granting of options to purchase Common Shares. The exercise price of the stock options granted under this amended plan is not lower than the closing price of the Common Shares on the TSXV at the close of markets the day preceding the grant. The maximum number of Common Shares that may be issued upon exercise of options granted under the amended stock option plan was increased from 5,494,209 representing 15% of the issued and outstanding Common Shares as of June 27, 2018, to 11,719,910 representing 15% of the issued and outstanding Common Shares o as of April 9, 2019. The terms and conditions for acquiring and exercising options are set by the Corporation’s Board of Directors in accordance with and subject to the terms and conditions of the stock option plan, and have a contractual life of 10 years.
The total number of shares issued to any one consultant within any twelve-month period cannot exceed 2% of the Corporation’s total issued and outstanding Common Shares (on a non-diluted basis). The Corporation is not authorized to grant within any twelve-month period such number of options under the stock option plan that could result in a number of Common Shares issuable pursuant to options granted to (a) related persons exceeding 2% of the Corporation’s issued and outstanding Common Shares (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve-month period exceeding 2% of the Corporation’s issued and outstanding Common Shares (on a non-diluted basis) on the date an option is granted.
The following tables summarize information about activities within the stock option plan:
| Number of options | Weighted average exercise price | Weighted average grant date fair value | ||||||||||
| CAD $ | CAD $ | |||||||||||
| Outstanding, March 31, 2018 | 2,284,388 | 1.81 | 1.16 | |||||||||
| Granted | 2,173,523 | 0.77 | 0.51 | |||||||||
| Exercised | (4,167 | ) | 0.77 | 0.55 | ||||||||
| Forfeited | (407,067 | ) | 1.84 | 1.21 | ||||||||
| Outstanding, March 31, 2019 | 4,046,677 | 1.25 | 0.81 | |||||||||
| Granted | 6,140,517 | 0.85 | 0.85 | |||||||||
| Exercised | (54,625 | ) | 1.11 | 0.79 | ||||||||
| Forfeited | (188,583 | ) | 1.64 | 1.16 | ||||||||
| Expired | (7,500 | ) | 6.50 | 3.02 | ||||||||
| Outstanding, March 31, 2020 | 9,936,486 | 1.00 | 0.83 | |||||||||
| Exercisable at end of year | 3,172,234 | 1.34 | 0.98 | |||||||||
| March 31, 2020 | March 31, 2019 | |||||||
| Weighted average fair value of the options granted to employees and directors of the Corporation | CAD$0.85 | CAD$0.51 | ||||||
Stock-based compensation recognized under the stock option plan for the year ended March 31, 2020 was $442,975 (CAD $592,469) included in research and development expenses and $1,510,026 (CAD $2,021,361) included in general and administrative expenses (for the year ended March 31, 2019, amounted to $183,636 (CAD $240,802) included in research and development expenses and $593,555 (CAD $779,059) included in general and administrative expenses). As of March 31, 2020, there was $1,992,002 (CAD $ 2,801,154) (as of March 31, 2019, $468,698)( CAD $608,840)) of total unrecognized compensation cost, related to non-vested share options, which is expected to be recognized over a remaining weighted average vesting period of 1.4 years (as of March 31, 2019, 1.25 years).
F-27
| 15. | Stock-based compensation (continued): |
| (a) | Corporation stock option plan (continued): |
A summary of the non-vested stock option activity and related information for the Corporation’s stock options granted is as follows:
| Number of options | Weighted average grant date fair value | |||||||
| CAD ($) | ||||||||
| Non- vested, March 31, 2019 | 2,433,477 | 1.05 | ||||||
| Options granted | 6,140,517 | 0.85 | ||||||
| Options vested | (1,798,075 | ) | 1.19 | |||||
| Options forfeited and cancelled | (11,667 | ) | 1.04 | |||||
| Non- vested, March 31, 2020 | 6,764,252 | 0.83 | ||||||
The fair value of options granted was estimated using the Black-Scholes option pricing model, resulting in the following weighted average assumptions for options granted during the periods ended:
| March 31, 2020 CAD Weighted average | March 31, 2019 CAD Weighted average | |||||||
| Exercise price | $ | 0.85 | $ | 0.77 | ||||
| Share price | $ | 1.09 | $ | 0.73 | ||||
| Dividend | — | — | ||||||
| Risk-free interest | 0.88 | % | 2.21 | % | ||||
| Estimated life (years) | 5.71 | 5.68 | ||||||
| Expected volatility | 99.11 | 86.10 | ||||||
The following tables summarize the status of the outstanding and exercisable options of the Corporation:
| March 31, 2020 | ||||||||||||||
| Exercise price CAD | Weighted average remaining contractual life |
Number of options outstanding |
Number of options exercisable |
|||||||||||
| $ | 0.53 | 10.00 | 3,836,000 | - | ||||||||||
| $ | 0.77 | 8.25 | 1,862,106 | 1,012,674 | ||||||||||
| $ | 0.78 | 8.48 | 200,000 | 100,000 | ||||||||||
| $ | 0.91 | 8.66 | 50,000 | 20,833 | ||||||||||
| $ | 1.28 | 9.04 | 1,991,059 | 540,573 | ||||||||||
| $ | 1.46 | 9.24 | 150,000 | 37,500 | ||||||||||
| $ | 1.56 | 3.11 | 525,000 | 525,000 | ||||||||||
| $ | 1.65 | 6.90 | 123,333 | 123,333 | ||||||||||
| $ | 1.77 | 7.20 | 747,500 | 498,333 | ||||||||||
| $ | 1.99 | 3.15 | 265,700 | 265,700 | ||||||||||
| $ | 2.82 | 9.63 | 150,000 | 12,500 | ||||||||||
| $ | 4.50 | 2.16 | 22,500 | 22,500 | ||||||||||
| $ | 4.80 | 2.38 | 13,288 | 13,288 | ||||||||||
| 9,936,486 | 3,172,234 | |||||||||||||
F-28
| 15. | Stock-based compensation (continued): |
| (a) | Corporation stock option plan (continued): |
Stock-based compensation payment transactions and broker warrants:
The fair value of stock-based compensation transactions is measured using the Black-Scholes option pricing model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility for a duration equal to the weighted average life of the instruments, life based on the average of the vesting and contractual periods for employee awards as minimal prior exercises of options in which to establish historical exercise experience; contractual life for broker warrants), and the risk-free interest rate (based on government bonds). Service and performance conditions attached to the transactions, if any, are not taken into account in determining fair value. The expected life of the stock options is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility over a period similar to the life of the options is indicative of future trends, which may also not necessarily be the actual outcome.
| (b) | Corporation equity incentive plan: |
The Corporation established an equity incentive plan for employees, directors and consultants. The plan provides for the issuance of restricted share units (RSUs), performance share units, restricted shares, deferred share units and other stock-based awards, subject to restricted conditions as may be determined by the Board of Directors. There were no such awards outstanding as of March 31, 2020 and March 31, 2019, and no stock-based compensation was recognized for the period ended March 31, 2020 and March 31, 2019.
| 16. | Loss per share: |
Diluted loss per share was the same amount as basic loss per share, as the effect of options, RSUs and warrants would have been anti-dilutive, as the Corporation has incurred losses in each of the periods presented. All outstanding options, RSUs and warrants could potentially be dilutive in the future.
| 17. | Supplemental cash flow disclosure: |
| (a) | Changes in working capital items: |
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Receivables | 581 | (620 | ) | |||||
| Prepaid expenses | (185 | ) | (530 | ) | ||||
| Trade and other payables | (3,389 | ) | 7,444 | |||||
| Total changes in working capital items | (2,993 | ) | 6,294 | |||||
| (a) | Non-cash transactions: |
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Issuance of shares for interest on convertible debt | - | 90 | ||||||
| Issuance of broker warrants included in net proceeds from public offering | - | 221 | ||||||
| Interest receivable included in receivables | 11 | 72 | ||||||
Shares issued as settlement | 738 | - | ||||||
| Deferred financing costs reclassified to Equity | 40 | - | ||||||
| Fair value of derivative warrants liability reclassified to equity | 10,691 | 550 | ||||||
| Equipment included in trade and other payables | - | 9 | ||||||
| Interest payable included in trade and other payables | - | 30 | ||||||
F-29
| Acasti pharma inc. |
(Expressed in thousands of U.S. dollars except share data) |
| 18. | Income taxes: |
Reconciliation of effective tax rate:
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Loss before income taxes | (25,513 | ) | (39,366 | ) | ||||
Basic combined Canadian statutory income tax rate 1 | 26.58 | % | 26.68 | % | ||||
| Computed income tax recovery | (6,781 | ) | (10,503 | ) | ||||
| Increase resulting from: | ||||||||
| Non-deductible stock-based compensation | 519 | 207 | ||||||
| Non-deductible change in fair value of warrants | 205 | 1,266 | ||||||
| Change in valuation allowance | 6,004 | 8,839 | ||||||
| Other – Foreign exchange | 20 | 36 | ||||||
| Other | 33 | 155 | ||||||
| Total tax (recovery) expense | 0 | 0 | ||||||
1 The Canadian combined statutory income tax rate has decreased due to a reduction in the provincial statutory income tax rate.
At March 31, 2020 and 2019, the net deferred tax assets have not been recognized in these financial statements. A valuation allowance is recognized to reduce the deferred tax assets as it is more likely than not that a tax benefit will not be realized.
Net deferred income tax assets as of March 31, 2020 and 2019 were comprised of the following:
| March 31, 2020 | March 31, 2019 | |||||||
| $ | $ | |||||||
| Deferred tax assets | ||||||||
| Tax losses carried forward | 22,052 | 17,750 | ||||||
| Research and development expenses | 4,544 | 4,017 | ||||||
| Property, plan and equipment | 324 | 254 | ||||||
| Intangible assets | 1 | (178 | ) | |||||
| Financing expenses | 998 | 1,387 | ||||||
| Tax credit carry forwards | 2,468 | 2,459 | ||||||
| Other temporary differences | 76 | 283 | ||||||
| Deferred tax assets | 30,463 | 25,972 | ||||||
| Deferred tax liabilities | ||||||||
| Tax basis of unsecured convertible debentures in excess of carrying value | - | (10 | ) | |||||
| Deferred tax liabilities | - | (10 | ) | |||||
| Valuation allowance | (30,463 | ) | (25,962 | ) | ||||
| Net deferred tax assets | - | - | ||||||
F-30
| 18. | Income taxes (continued): |
As at March 31, 2020, the amounts and expiry dates of tax attributes and temporary differences, which are available to reduce future years’ taxable income, were as follows:
| March 31, 2020 | ||||||||
| Federal | Provincial | |||||||
| $ | $ | |||||||
| Tax losses carried forward | ||||||||
| 2028 | 508 | 508 | ||||||
| 2029 | 1,157 | 1,152 | ||||||
| 2030 | 1,473 | 1,467 | ||||||
| 2031 | 1,609 | 1,594 | ||||||
| 2032 | 1,318 | 1,298 | ||||||
| 2033 | 2,559 | 2,559 | ||||||
| 2034 | 3,268 | 3,171 | ||||||
| 2035 | 3,907 | 3,907 | ||||||
| 2036 | 5,749 | 5,661 | ||||||
| 2037 | 356 | 352 | ||||||
| 2038 | 12,331 | 12,281 | ||||||
| 2039 | 28,811 | 28,773 | ||||||
| 2040 | 20,311 | 20,311 | ||||||
| 83,357 | 83,034 | |||||||
| Research and development expenses, without time limitation | 16,698 | 17,732 | ||||||
| Tax credit carry forwards | 2,468 | - | ||||||
| Other deductible temporary differences, without time limitation | 5,282 | 5,282 | ||||||
Unrecognized tax benefits
The following table summarizes the activity related to our gross unrecognized tax benefits for the years ended March 31, 2020 and 2019:
| March 31, 2020 |
March 31, 2019 |
|||||||
| $ | $ | |||||||
| Beginning of year: | ||||||||
| Increase (decrease) resulting from: | ||||||||
| Positions taken in the current year | 164 | - | ||||||
| Change in valuation allowance | (164 | ) | - | |||||
| End of year | - | - |
The Corporation does not expect a significant change to the amount of unrecognized tax benefits over the next 12 months. However, any adjustments arising from certain ongoing examinations by tax authorities could alter the timing or amount of taxable income or deductions, of the allocation of income among tax jurisdictions, and these adjustments could differ from the amount accrued.
The Corporation’s federal and provincial income tax returns filed for all years remain subject to examination by the taxation authorities.
F-31
| 19. | Financial instruments: |
| (a) | Concentration of credit risk: |
Financial instruments that potentially subject the Corporation to a concentration of credit risk consist primarily cash and cash equivalents and investments. Cash and cash equivalents and investments are all invested in accordance with the Corporation’s Investment Policy with the primary objective being the preservation of capital and the maintenance of liquidity, which is managed by dealing only with highly rated Canadian institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents the Corporation’s credit exposure at the reporting date.
| (b) | Foreign currency risk: |
The Corporation is exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of the Corporation's business transactions denominated in currencies other than the Corporations functional currency of the Canadian dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in the Corporation's operating results. The Corporation does not use derivative instruments to hedge exposure to foreign exchange risk. The fluctuation of the U.S. dollar in relation to the Canadian dollar and other foreign currencies will consequently have an impact upon the Corporation’s net loss.
The operating results and financial position of the Corporation are reported in U.S. dollars (reporting currency) in the Corporation’s financial statements.
| (c) | Liquidity risk: |
Liquidity risk is the risk that the Corporation will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Corporation manages liquidity risk through the management of its capital structure and financial leverage. It also manages liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves the Corporation's operating budgets, and reviews material transactions outside the normal course of business. Refer to Note 2(c).
The Corporation’s financial liabilities obligations include trade and other payables, which fall due within the next 12 months in addition to the warrant derivatives that fall due beyond 12 months and are likely to be settled by the Corporation’s equity.
| 20. | Commitments |
Research and development contracts and contract research organizations agreements:
The Corporation utilizes contract manufacturing organizations related to the development and production of clinical material and clinical research organizations to perform services related to the Corporation’s clinical trials. Pursuant to these agreements with manufacturing and contract research organizations, the Corporation has the right to terminate the agreements either without penalties or under certain penalty conditions. There are no penalties to be incurred in any open contracts.
RKO Supply Agreement:
On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antartic AS (”Aker”), to purchase raw krill oil product (RKO) for a committed volume of commercial starting material for CaPre for a total value of $3.1 million (take or pay). The delivery of the product has been established following a calendar year basis and it must be completed in the 4th calendar quarter of 2021. As at March 31, 2020, the remaining balance of the commitment with Aker amounts to $2.8 million.
| 21. | Comparative figures |
Certain comparative figures in the year ended March 31, 2019 have been adjusted, in order to conform to US GAAP. Adjustments included certain reclassifications within equity for certain warrants, the recognition of deferred tax on legacy transfers of license from Neptune that were subject to an initial recognition exemption under IFRS and different classifications within the statement of cash flows for treatment of interest expense and income.
F-32
| 22. | Subsequent events |
ATM Program
Subsequent to March 31, 2020, the Corporation sold a total of 2,278,936 Common Shares through the ATM program, for net proceeds of approximately $1.8 million (net of commissions paid for approximately $0.08 million). The shares were sold at the prevailing market prices which resulted in an average price of approximately $0.81 per share.
F-33